| Posted: March 31, 2010 |
Switched-on color-changing polymers |
|
(Nanowerk News) Azulene is an aromatic molecule with a rare, deep-blue color that sets it apart from other organic compounds. Recognized for over 500 years as a natural pigment, azulene’s unusual color results from an electronic interplay between the two main structural components, fused five- and seven-membered conjugated carbon rings. Recently, scientists have begun to exploit azulene’s optical abilities by incorporating it into materials such as liquid crystals and conductive polymers.
|
|
Now, Jianwei Xu, Chaobin He and co-workers from the A*STAR Institute of Materials Research and Engineering, in collaboration with researchers from Nanyang Technological University in Singapore, have combined azulene with a polycyclic organic molecule called fluorene to produce a series of polymers that can change color on demand using either a chemical or electronic stimulus ("Synthesis, Electronic, and Emission Spectroscopy, and Electrochromic Characterization of Azulene-Fluorene Conjugated Oligomers and Polymers"). This discovery may usher in a new generation of ‘smart’ materials, such as windows that can change from clear to opaque with the flick of a switch.
|
|
Fluorene, composed of two benzene rings fused onto a pentagonal hydrocarbon, has well-known fluorescent properties. According to Xu, the research team chose to combine this molecule with azulene because both units lie in the same physical plane after binding; this geometry ensures strong molecule-to-molecule interactions. Furthermore, fluorene can be easily modified so that it becomes soluble in organic solvents or water—an essential feature for processing and manufacturing.
|
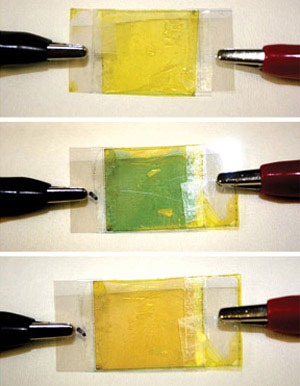 |
| Fig. 1: New azulene–fluorene polymer films can reversibly change color from yellow to green in response to an applied voltage. (Reproduced from Ref. 1 © 2009 American Chemical Society)
|
|
The researchers first linked the azulene and fluorene units into short chains called oligomers through a palladium-catalyzed reaction known as a Suzuki cross-coupling. Repeating this procedure produced long azulene–fluorene polymers with high molecular weights. Xu says that these polymers are relatively easy to make after the correct starting materials are synthesized.
|
|
Using a chemical agent called trifluoroacetic acid, the researchers could directly change the fluorescence and visible color of azulene–fluorene polymers in liquid solutions. Trifluoroacetic acid adds hydrogen atoms to the azulene rings, and theoretical calculations revealed that this chemical transformation shifts the internal energy levels of the polymer, initiating new optical behavior.
|
|
Finally, the researchers created the first azulene-based electrochromic device—a material that changes color instantly and reversibly through electricity—by spinning the polymers into thin films and sandwiching them between transparent electrodes. Because azulene readily releases or accepts electrons, even moderate electric potentials could switch the film color from green to yellow and back again (Fig. 1). Currently, the researchers are working to improve the performance of these colorful materials by incorporating new molecular units containing nitrogen and sulfur atoms.
|

