| Posted: April 14, 2010 |
Cell adhesion: Sticky predictions |
|
(Nanowerk News) The binding of cells onto other cells, or onto the extracellular matrix, is a vital but extremely complex biological process. Sometimes the adhesive bonds should be strong to provide structural robustness in tissues, while at other times they should be weaker to allow more movement.
|
|
Measuring cell adhesion is therefore essential for understanding biological processes including the internal spread of disease. Since measuring adhesion strength to a high degree of accuracy has proved difficult, YongWei Zhang and co-workers at the Institute of High Performance Computing of A*STAR, Singapore, Brown University, USA, and the National University of Singapore have developed a computer model aimed at making the measurements more precise ("A computational modeling for micropipette-manipulated cell detachment from a substrate mediated by receptor–ligand binding").
“We investigated how a cell, which is coated with mobile receptors that can diffuse in the cell wall, would adhere to and detach from a substrate coated with ligands,” explains Zhang.
|
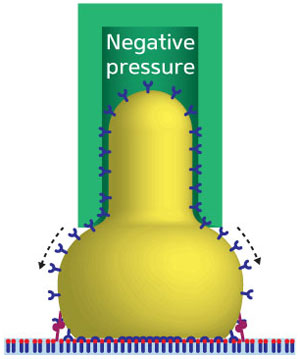 |
| Fig. 1: Schematic representation of a micropipette (green) used to measure the adhesion of a cell (yellow) to a substrate (light blue). Receptors (blue) migrate around the cell membrane to improve the binding to ligands (red) on the substrate. Directly beneath the middle of the cell, the increased receptor density and saturated bonds at equilibrium distance mean there is no force. Either side of this, the receptor-ligand pairs are partially bonded so there is a strong, specific force.
|
|
Experimental attempts to measure cell adhesion have included atomic force microscopy, optical traps and centrifugation. However, Zhang and his co-workers focused on a method whereby a cell is picked up in a micropipette, then allowed to adhere to another surface (Fig. 1). The cell is then pulled away from the surface while measuring the forces on the micropipette.
|
|
In their model, the researchers included not only the chemical reactions for the interacting receptors and ligands, but also weaker non-specific binding processes such as Van der Waals, or electrostatic, forces. Furthermore, they modeled the diffusion of receptors within the cell membrane and stresses in the membrane that may cause bonds to rupture.
|
|
The researchers found that after the first receptors bound to surface ligands, the non-specific forces attracted other receptors around the membrane, which were then more likely to bind to ligands. This created a larger contact area and caused deformation of the cell.
|
|
Most importantly, Zhang and co-workers’ model showed that as a cell is drawn upwards with the micropipette, it shifted slightly out of the pipette tube, which greatly affected binding kinetics and introduced a measurement error.
|
|
Application of these findings could greatly improve estimates of adhesion strength and lead to useful medical applications. “For example, to begin the process of metastasis [the spread of cancers from one organ to another], a malignant cell must first break away from the cancerous tumor,” says Zhang. “These unattached cells can neither reproduce nor grow, so in order to survive and proliferate, they have to bind to the extracellular matrix or other cells. Our research could improve our understanding of the metastasis process.”
|

