| Posted: March 7, 2007 |
The principles of molecule-selective nanovalves in carbon nanotubes |
|
(Nanowerk News) Associate Professor Yutaka Maniwa's group with the Graduate School of Science and Engineering of Tokyo Metropolitan University and Hiromichi Kataura, chief of the Self-Assembled Nano-Electronics Group at the Nanotechnology Research Institute of the National Institute of Advanced Industrial Science and Technology (AIST) jointly demonstrated the adsorption of water molecules into a Single-Walled Carbon Nanotube (SWCNT) under different gas ambiences and discovered the "exchange transition" phenomenon involving ambient gas and water molecules.
|
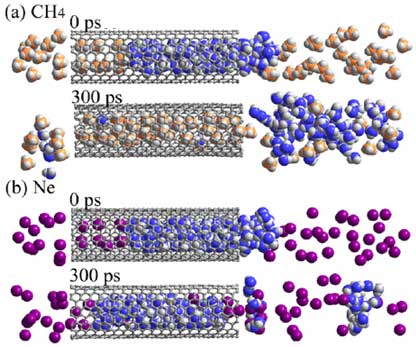 |
| Exchange transition for CH4 and Ne. (a), (b); molecular dynamics simulations for the coexistent systems of water and CH4 (a) and Ne (b) molecules. Initially the water clusters are located inside SWCNT. After 300 ps, CH4 molecules enter the SWCNT, although Ne molecules cannot enter. (Image: AIST)
|
|
The "exchange transition"-characterized as an exchange between molecules of water inside the SWCNT and ambient gas molecules -was verified for seven types of ambient gas: argon, krypton, oxygen, nitrogen, methane, ethane, and carbon dioxide. The conditions for occurrence of exchange transition depend on the type of gas. For example, with methane at one atmosphere and -30°C or below, water molecules are expelled from the SWCNT and replaced by methane molecules which penetrate into the SWCNT. In contrast, when helium, hydrogen, or neon was used, water molecules remained stable inside the SWCNT at temperatures of -170°C or below. Using this phenomenon, the water-filled SWCNT can be used as a molecule-selective nanovalve.
|
|
In addition, the sudden change in electrical resistance of the SWCNT film due to the exchange transition can be used to create a new gas sensor that permits the selection of gases without any special chemical treatment, coating, or the like. Tokyo Metropolitan University and AIST plan to put the gas sensor and the molecule-selective nanovalve to practical use and invite the participation of companies possessing related technologies.
|
|
The results of the present research were published in the on-line version of the scientific journal Nature Materials ("Water-filled single-wall carbon nanotubes as molecular nanovalves") on January 21, 2007. Part of it has received support from the Japan Science and Technology Agency/Core Research for Evolutional Science and Technology (CREST).
|

