| Posted: April 16, 2010 |
In the electron cloud |
|
(Nanowerk News) The chemistry between atoms and molecules is strongly determined by their outer electron orbitals, or clouds, which participate in chemical processes. A team from three Japanese research institutes has now developed a method that can measure the three-dimensional shape and dynamics of an electron cloud ("Molecular Frame Image Restoration and Partial Wave Analysis of Photoionization Dynamics of NO by Time-Energy Mapping of Photoelectron Angular Distribution").
|
|
“The shape of an electron cloud is at the heart of intermolecular interactions that lead to beautiful chemistry,” comments Toshinori Suzuki from the RIKEN Advanced Science Institute in Wako, who led the research team.
|
|
Measuring the dynamics of an electron cloud is challenging because molecules in gases and liquids always move randomly; this makes it difficult to take a ‘snapshot’ of movement averaged over many molecules at a specific moment in time. However, the excitation of nitric oxide (NO) by a polarized laser beam can align those molecules along one axis, so that the measurement of their outer electron cloud becomes possible.
|
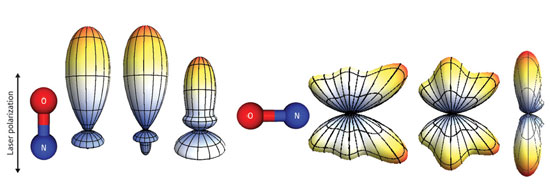 |
| Figure 1: Experimental measurement of the temporal evolution cycle (left to right) of the outer electron cloud of an NO molecule. The arrows show the polarization of the laser beam that aligns the molecules during the experiment.
|
|
To detect the shape of the outer electron cloud of an NO molecule aligned by the first laser pulse, Suzuki and colleagues released the electrons from the molecule using a second laser pulse. They then applied an electric field to accelerate and project the expanding electron cloud onto a fluorescent screen where it was visualized as a direct representation of the original electron distribution (Fig. 1). The researchers then used computer algorithms, similar to those from computer tomography, to construct a three-dimensional picture from the two-dimensional representation.
|
|
Fundamental quantum mechanical principles limit the degree to which the molecules can be aligned by the laser pulse, Suzuki notes. This means that there is always unavoidable blurring in the reconstructed three-dimensional image. Removing this blurring in the final images was the most difficult part of the process, he says.
|
|
Suzuki and colleagues therefore analyzed how a three-dimensional image changes when the molecules rotate out of alignment. By correcting these misalignment effects, they eventually succeeded in perfectly sharpening the image.
|
|
The team’s algorithm can visualize the outer electron cloud of a molecule at rest, but the challenge now is to map the rapid changes that occur during chemical reactions. “The NO molecule was just a testing ground,” explains Suzuki. “Our main target is more complex molecules and their chemical reactions in response to light of different color.” Outlining his future vision, Suzuki says he would like to study the mechanism of photodamage to DNA starting with real-time observations of electron motions in their constituent base molecules.
|

