| Posted: April 28, 2010 |
Microfluidic mixing and the formation of nanoscale lipid vesicles |
|
(Nanowerk News) Pop a bubble while washing the dishes and you’re likely to release a few drops of water trapped when the soapy sphere formed. A few years ago, researchers at the National Institute of Standards and Technology (NIST) and the University of Maryland (UM) pioneered a method ("Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing") using a microscopic fluidic (microfluidic) device that exploits the same principle to create liquid-filled vesicles called liposomes from phospholipids, the fat complexes that are the building blocks for animal cell membranes. These structures are valued for their potential use as agents to deliver drugs directly to cancers and other disease cells within the body.
|
 |
| Flexing muscle: IPMC actuator bending under an applied electrical voltage. As the polarity of the 3-volt potential is switched, the actuator bends back and forth.
|
|
Widespread application of liposomes as artificial drug carriers has been hindered by a number of limiting factors such as inconsistency in size, structural instability and high production costs. In a new study ("Microfluidic mixing and the formation of nanoscale lipid vesicles"), the NIST and UM researchers have detailed how the operation of their liposome manufacturing technique—known as COMMAND for COntrolled Microfluidic Mixing And Nanoparticle Determination—in order to maximize its effectiveness. Their goal was to better understand how COMMAND works as it produces liposomes with diameters controlled from about 50 to 150 nanometers (billionths of a meter) that are consistently uniform in size and inexpensively produced in what might be called an “assembly-line-on-a-microchip.”
|
|
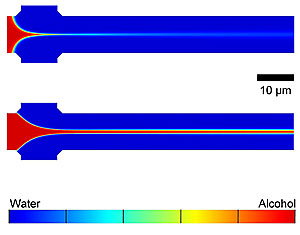
The researchers fabricate the COMMAND microfluidic devices by etching tiny channels into a silicon wafer with the same techniques used for making integrated circuits. In COMMAND, phospholipid molecules dissolved in isopropyl alcohol are fed via a central inlet channel into a “mixer” channel and “focused” into a fluid jet by a water-based solution (that in production would carry a drug or other cargo for the vesicles) added through two side channels. The components blend together as they mix by diffusion across the interfaces of the flowing fluid streams, directing the phospholipid molecules to self-assemble into nanoscale vesicles of controlled size. Different microfluidic device designs and fluid flow conditions were tested to investigate their role in producing liposomes.
|
|
The research team found that their liposome manufacturing process fundamentally depends on the flow and mixing of the fluid streams. The size of the liposomes can be “tuned” by manipulating the fluid flow rates, which in combination with the dimensions of the microfluidic device, determines the resulting mixing conditions. A tightly focused stream of phospholipid-carrying alcohol flowing at a slow rate tends to mix quickly with the buffer at the beginning of the mixing channel and forms small vesicles. A loosely focused stream flowing at a fast rate travels farther down the length of the mixing channel, allowing more mixing time and yielding larger vesicles.
|
|
The geometry of the channels plays an additional role, the researchers noted, in regulating the speed of production and the quantity and concentration of liposomes manufactured. This may be important for future clinical applications of liposomes as well as the integration of COMMAND into more complicated microchip systems for health care.
|

