| Posted: July 20, 2010 |
Synthetic strategy allows the manipulation of ordering transitions in block copolymer nanostructures |
|
(Nanowerk News) How do you make a material that has the elasticity of a rubber band and the thermal insulation of a Styrofoam cup? Connect two distinct polymer chains - poly(isoprene) and poly(styrene) - end to end like a series of children's building blocks. The result is an appropriately named "block copolymer" that boasts the properties of both materials and is commonly used in the tires of automobiles and the soles of athletic shoes.
|
|
But the most impressive trait of a block copolymer is its ability to self-assemble. Imagine, for example, dropping a mixture of poly(isoprene) and poly(styrene) on the floor. The two incompatible blocks will 'phase separate' like oil and water. However, connect the ends of the two polymers together and the material usually can assemble into a well-defined material with nanoscale structure.
|
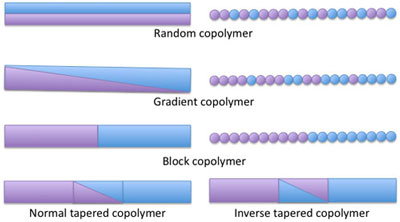 |
| Cartoon of density profile and segment distribution along model polymer chains as a function of position, where purple represents isoprene and blue represents styrene, illustrating the difference between random, gradient, block, and tapered block copolymers.
|
|
The natural assembly is extremely valuable for emerging nanoscale applications, including materials for fuel cells, lithium ion batteries, and organic photovoltaics.
|
|
Block copolymers can create well-defined nanostructures without traditional nanoscale processing, said University of Delaware researcher Thomas Epps. The usage of block copolymers can help augment or replace lithographic and other techniques, meaning one can make nanoscale materials without the expensive tools.
|
|
In order to take full advantage of a block copolymer's molecular architecture, researchers are looking for ways to control the interactions between polymer blocks through means such as high temperatures and selective solvents.
|
|
"When I make a copolymer of a certain composition and molecular weight, I have typically locked myself into a processing temperature based on those chemical factors," Epps said. "We want to find a way to tune these materials so that, no matter what blocks are being combined, we can control the processing conditions."
|
|
To do this, Epps and fellow University of Delaware researchers Nripen Singh and Maeva Tureau are exploring tuning with tapering, which increases the compatibility of the polymer by smoothing out the chemical interface between the two blocks.
|
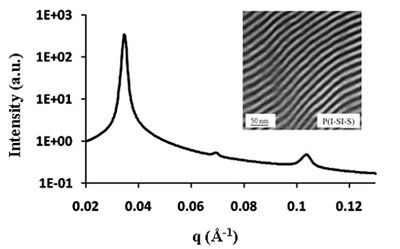 |
| Synchrotron-SAXS data for a P(I-SI-S) tapered diblock copolymer. Specimens were annealed at 210 oC and then cooled to room temperature for data acquisition. The integral moduli values are characteristic of two-domain lamellae. The inset shows a transmission electron microscopy image of a P(I-SI-S) specimen. The sample is stained by OsO4 vapor to enhance contrast.
|
|
Synchrotron-SAXS data for a P(I-SI-S) tapered diblock copolymer. Specimens were annealed at 210 oC and then cooled to room temperature for data acquisition. The integral moduli values are characteristic of two-domain lamellae. The inset shows a transmission electron microscopy image of a P(I-SI-S) specimen. The sample is stained by OsO4 vapor to enhance contrast.
|
|
Tapering conceptualized: think of a block of purple beads linked to a block of blue beads. A regular block copolymer will have a sharp interface separating the two types of beads. In a tapered block copolymer, a region between the two blocks has been inserted such that the number of purple beads will slowly decrease as the number of blue beads increases. In an inverse tapered block copolymer, the opposite occurs (see figure).
|
|
Tapering the interface is thought to reduce the "penalty" of mixing between the two very different blocks. The result is a lower processing temperature, which is easier and cheaper to achieve, and greater chemical compatibility.
|
|
To test this idea, the researchers synthesized a number of tapered poly(isoprene-b-styrene) block copolymer samples at the University of Delaware: samples with different taper lengths (from 15-35 percent tapered material) with either normal or inverse tapering.
|
|
The samples were then heated and cooled while researchers observed the changes in the material with small-angle x-ray scattering at the NSLS (beamline X27C) and Argonne's Advanced Photon Source, and transmission electron microscopy and dynamic mechanical analysis at the University of Delaware.
|
|
Their results, which appeared in Soft Matter ("Manipulating ordering transitions in interfacially modified block copolymers"), proved that researchers can tune the compatibility of block copolymers (via tapering) with no detriment to the ordering or mechanical properties of the material. The research also revealed that inverse tapering gives the greatest increase in compatibility.
|
|
"The inverse tapering forces more unfavorable interactions in the as-synthesized copolymer so the penalty for creating more interactions through self-assembly or mixing is smaller," Epps said.
|


