| Posted: July 21, 2010 |
Nanomaterials: Twist-on caps |
|
(Nanowerk News) Single-walled carbon nanotubes (SWCNTs) are versatile nanomaterials made of rolled-up honeycomb-like sheets of carbon atoms. Depending on the direction and degree of twisting of these sheets, nanotubes can behave as metals or semiconductors, which is a potential boon for the development of ultrasmall electromagnetic devices. However, the exact mechanism that controls this twisting, known as chirality, has so far eluded materials scientists.
|
|
Now, using quantum calculations, a team led by Man-Fai Ng and Shuo-Wang Yang from the A*STAR Institute of High Performance Computing in Singapore has revealed the role of single carbon atoms and carbon–carbon (C2) dimer molecules in the 'growth' mechanism of chiral SWCNTs ("The Mechanism of Single-Walled Carbon Nanotube Growth and Chirality Selection Induced by Carbon Atom and Dimer Addition").
|
|
During the synthesis of SWCNTs, nanotubes are often capped by hemispherical arrays of pentagonal and hexagonal carbon rings. As nanotube caps have an important role in determining chirality, Yang and his team undertook a systematic approach to determine how the 'armchair' and 'near-armchair' types of caps (Fig. 1) evolve during SWCNT growth upon interacting with single carbon atoms and C2 dimers without catalysts.
|
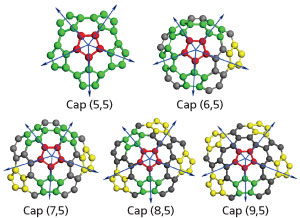 |
| Fig. 1: Molecular representations of armchair (5,5) and near-armchair caps (n,5) (n,6–9). The blue arrows show the pentagons and the direction of pentagon movement. Red represents the carbon atoms forming the central pentagon, green represents carbon atoms of the peripheral pentagons in the armchair cap, yellow represents carbon pentagons formed during growth, and gray represents other carbon atoms. (Reproduced, with permission, from Ref. 1 © 2010 American Chemical Society)
|
|
"To uncover the precise reaction mechanism and [reaction] paths, we performed a survey which provides the potential energy of each point around the reaction center during nanotube growth," explains Yang. Unlike other studies that have focused on finding transition states of reactions, Yang believes that his team's approach pinpoints the most favorable reaction paths.
|
|
After evaluating the energies of intermediate structures growing from an armchair-type cap, the researchers identified two competing reaction pathways that could affect nanotube twisting. In the first, single carbon atoms react with caps to change their chirality; in the second pathway, C2 dimers react with caps to induce tube growth.
|
|
The team's calculations revealed that single carbon atoms and C2 dimers would spontaneously attach to the pentagonal edges of the caps, suggesting that their relative abundance during growth stages could be an important factor in the synthesis of chiral nanotubes. This means that more single carbon atoms than C2 dimers would favor chirality change, whereas more C2 dimers than single atoms would facilitate growth.
|
|
The researchers also noted that single carbon atoms and C2 dimers would readily produce hexagons upon binding to the pentagon edges. The resulting near-armchair caps would then produce chiral caps that develop into chiral tubes through several successive additions of C2 dimers to form hexagons.
|
|
"We are currently working on growing the SWCNTS on metal substrates," says Yang. The team is also investigating the influence of catalyst nanoparticles on carbon cap formation.
|

