| Posted: July 23, 2010 |
Bright lights for live cells |
|
(Nanowerk News) Chemical reactions that create durable bonds between cells and fluorescent dyes are an attractive way to monitor biological functions in the body. However, they can also detrimentally modify key chemical groups on cell surfaces. Chemically binding fluorescent dyes to cells—with minimal impact on their original function—is now possible, thanks to a site-selective reaction developed by a team led by Yasuyoshi Watanabe and Tsuyoshi Tahara from the RIKEN Center for Molecular Imaging Science, Kobe ("Electrocyclization-Based Labeling Allows Efficient In Vivo Imaging of Cellular Trafficking").
|
|
Working closely with Katsunori Tanaka and Koichi Fukase from Osaka University, the researchers decided to use organic compounds called aldehydes as dye precursors as they readily react with nitrogen-containing functionalities, or amino groups, exposed at protein surfaces.
|
|
To assess the efficacy of their method, the team mixed the aldehydes with brain cancer cells in vitro for 10 minutes then compared them with typical amino reactive dye precursors known as succinimidyl esters (NHS) (Fig. 1). They discovered that the aldehyde precursors produced brighter fluorescence than the NHS dyes.
|
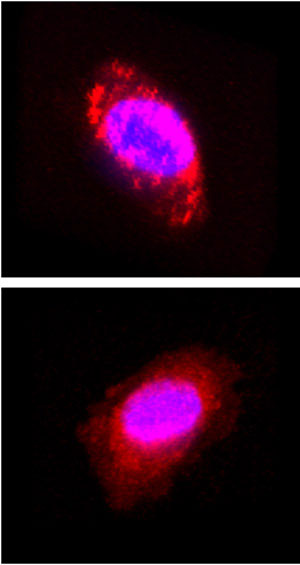 |
| Figure 1: Confocal microscopy images of a brain cancer cell labeled using aldehydes (top) and succinimidyl esters (bottom) as fluorescent dye precursors. Nuclei are shown in blue.
|
|
Confocal microscopy showed that the aldehydes reacted with amino groups of lysine amino acid residues on the cell surface and those of other cell membrane components, whereas the NHS dyes penetrated the cells. They confirmed this by treating the labeled cells with detergent: the aldehyde-derived labels washed off the cell surface, whereas their NHS counterparts remained in the cells. The aldehyde-derived labels also remained effective at exceptionally low concentrations, unlike the NHS-derived labels.
|
|
"In contrast to pre-existing cell labeling protocols, this reaction tightly anchors the labels to the surface of living cells within 10 minutes at 10 nM [dye] concentrations and with a very simple 'kit-like' operation," say Watanabe and Tahara. The team also observed that the brain cells maintained their ability to undergo cell division after labeling because of the mild reaction conditions.
|
|
The researchers also labeled lymphocytes, extracted from mice, with the fluorescent dyes and injected them into live mice for in vivo monitoring. They found that the labels clearly highlighted the trafficking of the cells into the organs of the mouse immune system. In particular, they noted that the cells gradually accumulated in the spleen and intestinal lymph nodes in six hours before disappearing from the spleen.
|
|
In addition to investigating potential clinical applications, the team is currently planning to apply their method to the synthesis of metal binding labels to introduce radioactive and magnetic properties into cells for imaging techniques such as positron emission tomography and magnetic resonance imaging.
|

