| Posted: August 4, 2010 |
Structural biology: Unsheathing cellular construction |
|
(Nanowerk News) An international team of molecular biologists led by Robert Robinson at the A*STAR Institute of Molecular and Cell Biology in Singapore has uncovered a mechanism for regulating the construction of actin filaments ("Structural characterization of a capping protein interaction motif defines a family of actin filament regulators"), which are the major component of the cellular skeleton and integral to many cellular processes. The mechanism centers around how capping protein (CP) covers and uncovers the active end of the filament. The team's findings also help explain how cells control important activities such as movement and endocytosis, where the outer membrane engulfs part of its surrounds.
|
|
Actin filaments are polymers of the protein actin. The ability to build or add to these chains of actin provides the driving force to shape many cellular processes. Actin adds freely to only one end of an existing filament, the 'barbed' end. Typically, this end is covered by CP to prevent uncontrolled filament extension.
|
|
Earlier work by other researchers has shown CP to be mushroom-shaped, binding to the barbed end of the actin filament via the surface of its 'mushroom cap'. Several molecules are known to inhibit capping, including the proteins CARMIL and CD2AP. Robinson and his co-workers from A*STAR and Washington University in the USA investigated the interaction between these two proteins and CP.
|
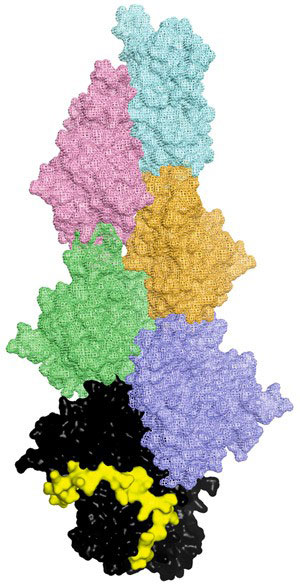 |
| Fig. 1: A model of a CP-capped actin filament. The uncapping motif (yellow) interacts with capping protein (black) to free the end of an actin filament (all other colors).
|
|
The region that binds to CP, known as the CP interaction motif, is similar in each of the proteins. By chopping CARMIL into fragments, the researchers were able to study exactly what sequence is necessary for binding and acting on CP, as well as the strength of the interaction. Using X-ray crystallography, they were able to determine that the proteins in the CP–protein complexes are bound to and wrapped around the stalk of CP (Fig. 1). This suggests that, rather than directly interfering in CP–filament bonding, the interaction acts remotely, changing the shape of CP and thereby causing it to release and fall away from the barbed end of the actin filament.
|
|
By searching among other proteins for the CP interaction motif, the researchers found a whole family of uncapping molecules including one, called WASHCAP, which also interacts with a complex that initiates the building of new filaments. WASHCAP could be used simultaneously to uncap existing filaments and stimulate new ones.
|
|
The team is now looking at other actin regulators. "Particularly, we would like to know how actin filaments in complex cells form three-dimensional structures and compare this to the structures formed in simpler cells," Robinson explains. "We hope to exploit these differences in the search for novel antibiotics."
|

