| Posted: September 1, 2010 |
Thermoelectrics: Unearthing hidden 'talent' |
|
(Nanowerk News) Thermoelectric materials convert temperature gradients to electric voltage and vice versa by causing charge carriers to diffuse from hot to cold parts of the material. They are used to power spacecraft, harness waste heat from sources including industrial plants and cars, generate electricity from solar heat, and provide low-cost cooling in consumer products. Good thermoelectric materials are normally semiconductors with implanted impurities, and their performance usually does not vary with the orientation of their crystal axes with respect to the temperature gradient.
|
|
Now, Khuong Ong and Ping Wu from the A*STAR Institute of High Performance Computing in Singapore and David J. Singh from Oak Ridge National Laboratory in the USA have uncovered two materials with previously unknown thermoelectric functionality ("Unusual Transport and Strongly Anisotropic Thermopower in PtCoO2 and PdCoO2").
|
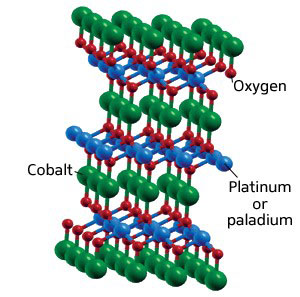 |
| Fig. 1: Crystal structure of delafossite oxides PtCoO2 and PdCoO2.
|
|
The two materials are platinum cobalt oxide (PtCoO2) and paladium cobalt oxide (PdCoO2), both with a 'delafossite' structure (Fig. 1) more commonly associated with oxides of copper and iron. The compounds are unique for two reasons: they are metallic rather than semiconducting, and they show strong thermoelectric properties when their crystal axes are oriented in a particular way with respect to the temperature gradient.
|
|
Because the effect was found in oxides, it is likely to be robust with respect to small changes in atomic bonding and could therefore have considerable practical utility. "These materials make very useful thermoelectrics," says Ong. "They can be used in highly oxidizing environments, since they are already oxides, and furthermore the underlying physics may also exist in other delafossite metals and semiconductors."
|
|
The research thus opens up a new area of thermoelectric research and introduces two new practical thermoelectric materials. The results are also of fundamental interest, because they provide a model for probing the fundamental limits of the theory of electron and heat transport in metals, according to the team. This is because PtCoO2 is close to the limit at which the spacing between atoms is similar to the average distance between electron scattering events. Electronic behavior in this circumstance, known as the Ioffe-Regel limit, is incompletely understood.
|
|
The results may have other scientific implications. For example, it is known that two-dimensional metals behave differently compared with three-dimensional metals. Because the two oxides studied by Ong and his co-workers are composed of flat sheets of platinum or paladium atoms separated by insulating sheets of cobalt oxide, their unusual behavior may be related to the dependence of electron transport on dimension. Effects due to the correlation of distant charges may also play a role.
|

