| Posted: Sep 22, 2010 |
|
New computer-tomography method visualizes nanostructure of bones
|
|
(Nanowerk News) A novel nano-tomography method developed by a team of researchers from the Technische Universitaet Muenchen, the Paul Scherrer Institute and the ETH-Zurich opens the door to computed tomography examinations of minute structures at nanometer resolutions. Three-dimensional detailed imaging of fragile bone structures becomes possible. Their first nano-CT images will be published in Nature on Sept. 23, 2010. This new technique will facilitate advances in both life sciences and materials sciences.
|
|
Osteoporosis, a medical condition in which bones become brittle and fragile from a loss of density, is among the most common diseases in aging bones: In Germany around a quarter of the population aged over 50 is affected. Patients' bone material shrinks rapidly, leading to a significantly increased risk of fracture. In clinical research to date, osteoporosis is diagnosed almost exclusively by establishing an overall reduction in bone density. This approach, however, gives little information about the associated, and equally important, local structure and bone density changes.
|
|
Franz Pfeiffer, professor for Biomedical Physics at the Technische Universitaet Muenchen (TUM) and head of the research team, has resolved the dilemma: "With our newly developed nano-CT method it is now possible to visualize the bone structure and density changes at high resolutions and in 3D. This enables us to do research on structural changes related to osteoporosis on a nanoscale and thus develop better therapeutic approaches."
|
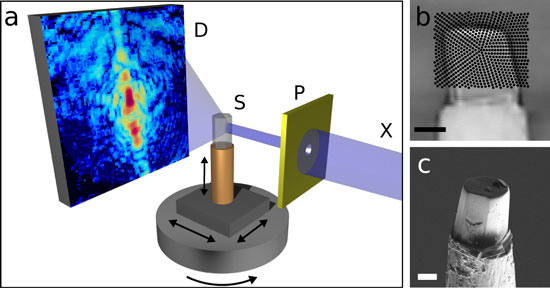 |
| Schematic of the new nano-CT method. The sample is scanned with an X-ray beam while the detector records a diffraction pattern for every beam position. The sample is then turned around its axis and scanned again, until a complete set of data is gathered for every angle. A high-resolution three-dimensional image of the sample is then computed from the hundreds of thousands of diffraction patterns by means of specially developed image reconstruction algorithms.
|
|
During development, Pfeiffer's team built on X-ray computed tomography (CT). The principle is well established – CT scanners are used every day in hospitals and medical practices for the diagnostic screening of the human body. In the process the human body is X-rayed while a detector records from different angles how much radiation is being absorbed. In principle it is nothing more than taking multiple X-ray pictures from various directions. A number of such pictures are then used to generate digital 3D images of the body's interior using image processing.
|
|
The newly developed method measures not only the overall beam intensity absorbed by the object under examination at each angle, but also those parts of the X-ray beam that are deflected in different directions – "diffracted" in the language of physics. Such a diffraction pattern is generated for every point in the sample. This supplies additional information about the exact nanostructure, as X-ray radiation is particularly sensitive to the tiniest of structural changes. "Because we have to take and process so many individual pictures with extreme precision, it was particularly important during the implementation of the method to use high-brilliance X-ray radiation and fast, low-noise pixel detectors – both available at the Swiss Light Source (SLS)," says Oliver Bunk, who was responsible for the requisite experimental setup at the PSI synchrotron facilities in Switzerland.
|
|
The diffraction patterns are then processed using an algorithm developed by the team. TUM researcher Martin Dierolf, lead author of the Nature article, explains: "We developed an image reconstruction algorithm that generates a high-resolution, three-dimensional image of the sample using over one hundred thousand diffraction patterns. This algorithm takes into account not only classical X-ray absorption, but also the significantly more sensitive phase shift of the X-rays."
|
|
|
|
A showcase example of the new technique was the examination of a 25-micrometer, superfine bone specimen of a laboratory mouse – with surprisingly exact results. The so-called phase contrast CT pictures show even smallest variations in the specimen's bone density with extremely high precision: Cross-sections of cavities where bone cells reside and their roughly 100 nanometer-fine interconnection network are clearly visible.
|
|
"Although the new nano-CT procedure does not achieve the spatial resolution currently available in electron microscopy, it can – because of the high penetration of X-rays – generate three-dimensional tomography images of bone samples," comments Roger Wepf, director of the Electron Microscopy Center of the ETH Zurich (EMEZ). "Furthermore, the new nano-CT procedure stands out with its high precision bone density measurement capacity, which is particularly important in bone research." This method will open the door to more precise studies on the early phase of osteoporosis, in particular, and evaluation of the therapeutic outcomes of various treatments in clinical studies.
|
|
The new technique is also very interesting for non-medical applications: Further fields of application include the development of new materials in materials science or in the characterization of semiconductor components. Ultimately, the nano-CT procedure may also be transferred to novel, laser-based X-ray sources, such as the ones currently under development at the Cluster of Excellence "Munich-Centre for Advanced Photonics" (MAP) and at the recently approved large-scale research project "Centre for Advanced Laser Applications" (CALA) on the TUM-Campus Garching near Munich.
|

