| Posted: Sep 24, 2010 |
|
Heads up, tails down
|
|
(Nanowerk News) The behavior of water molecules as they contact biological substances has long puzzled scientists. The first few layers of interfacial water can display complex arrangements that distinctly influence biochemical reactivity and function. Mapping these interfaces, however, is extremely difficult because chemical signatures of surface-bound water are often swamped by bulk liquid signals.
|
|
Now, researchers led by Tahei Tahara from the RIKEN Advanced Science Institute in Wako have developed a laser spectroscopy technique that conclusively determines the orientation of water molecules beneath charged lipid layers—the primary components of cell membranes ("Structure and Orientation of Water at Charged Lipid Monolayer/Water Interfaces Probed by Heterodyne-Detected Vibrational Sum Frequency Generation Spectroscopy").
|
|
Phospholipids are fatty acid molecules that contain two parts: hydrophobic 'tails' made of long hydrocarbon chains and hydrophilic 'heads' comprised of charged phosphate groups and other organic units. At the air–water interface, phospholipids spontaneously form into monolayer films, with their tails extending into the air and their heads immersed in water. The structure and orientation of water molecules below such monolayers has been a matter of controversy. Some investigators suggest that the partially positive-charged hydrogen atoms of water orientate 'up' or 'down' to align with the lipid head charge, while others suggest the opposite outcome.
|
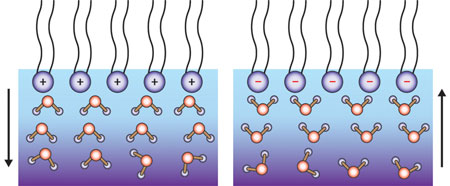 |
| Figure 1: Schematic diagram showing that, when the air–water interface is positively charged (top left), the water molecules (bottom) will orientate 'hydrogen-down' (left), and when it is negatively charged they are 'hydrogen-up' (right).
|
|
Tahara and colleagues resolved this debate by using an optical technique called heterodyne-detected vibrational sum frequency generation (HD-VSFG) spectroscopy, which has extremely high surface sensitivity. HD-VSFG combines two laser beams with different frequencies at an interface to generate a sum-frequency signal; when vibrations of surface molecules resonate with the applied laser, the sum-frequency signal rapidly shoots up—instantly identifying which chemicals are present. Because this signal originates from non-linear surface polarization effects, it contains only contributions from interfacial species. "HD-VSFG automatically probes the depths of water layers that are different from the bulk," says Tahara.
|
|
Determining the orientation of surface water required heterodyne detection, a method that determines the phase of weak signals via interference with a reference beam. According to Tahara, performing such measurements required precisely sensing changes to the signal light's optical phase—meaning the researchers had to control the laser beams with nanometer-scale accuracy.
|
|
The teams' experiments on three different lipid monolayers revealed that the interfacial structures are governed by the net charge of the heads: water hydrogen atoms pointed up with anionic lipid heads, and faced downwards in the presence of cationic lipids (Fig. 1). "This is totally different from the situation for reactions in aqueous solutions," says Tahara, who believes that the results will shed light on important reactions that take place at cell membranes, such as enzyme activation.
|

