| Posted: April 25, 2007 |
Nanotechnology's Trojan horse |
|
(Nanowerk News) A growing body of research has shown that nanoparticles can readily penetrate cells of various types. But it may not be the particles alone that cause trouble inside cells. A new study published today on Environmental Science & Technology's Research ASAP website suggests that toxicity is greatly increased by harmful metals that hitch a ride with nanoparticles ("Exposure of Engineered Nanoparticles to Human Lung Epithelial Cells: Influence of Chemical Composition and Catalytic Activity on Oxidative Stress").
|
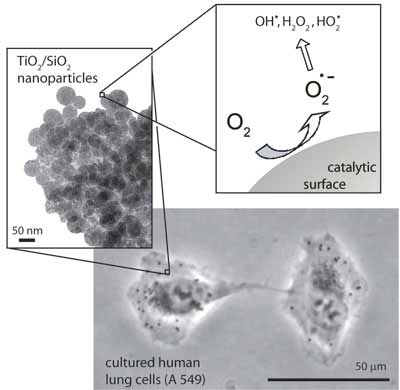 |
| When human lung epithelial cells (bottom) take up metal-containing nanoparticles (left), harmful molecules can be generated in reactions at the nanoparticle surface (right). (Images: Wendelin Stark)
|
|
Researchers at the Swiss Federal Institute of Technology (ETH) Zurich and the Swiss Federal Laboratories for Materials Testing exposed human lung epithelial cells to a range of metal-containing nanoparticles and measured levels of reactive oxygen species (ROS), chemicals that can be released by cells or generated by foreign materials. An excess of these reactive molecules can lead to oxidative stress and cellular damage, and toxicologists have identified ROS generation as a likely mechanism of nanoparticle toxicity. In cells exposed to cobalt oxide and manganese oxide nanoparticles at 30 parts per million, ROS generation was as much as 8 times greater than in control cells exposed to an equivalent amount of cobalt or manganese salts.
|
|
"What we see with the cobalt and the manganese is, if we take them as their chlorides, the cells don't like it, but they're not dying of the stuff either," says Wendelin Stark, head of the Functional Materials Laboratory at ETH Zurich and the paper's corresponding author. "They can defend themselves quite well, actually."
|
|
Stark, who has previously demonstrated nanoparticle uptake by human lung fibroblast cells, explains that cell membranes provide a selective barrier against ions, preventing the dissolved metal salts from entering. Once a metal-containing nanoparticle has penetrated a cell, however, metal ions can leach from the particle and generate ROS in the cell interior—in what Stark calls a "Trojan horse" mechanism. Mark Wiesner, professor of civil and environmental engineering at Duke University, finds it notable that measured levels of ROS generation were so much higher in cells exposed to the metal-oxide nanoparticles than in those exposed to the salts. But he believes that it would be helpful to have more detailed information about the types of ROS generated and their effect on the cells.
|
|
In addition to studying pure metal oxides, Stark and his colleagues experimented with 20–75-nanometer-sized silica nanoparticles containing 0.5 and 1.6 wt % titanium, iron, cobalt, and manganese—a choice of materials influenced by Stark's background in heterogeneous catalysis, in which transition metals are often used in redox reactions. The researchers were surprised to find that the in vitro behavior of nanoparticles containing the four different transition metals matched their relative activities in catalytic reactions. Titanium-doped particles caused the least ROS generation, as expected for a photocatalyst in the absence of light. Cobalt- and manganese-containing particles generated significantly more ROS than those doped with iron.
|
|
According to Greg Lowry, professor of civil and environmental engineering at Carnegie Mellon University, the researchers made a good case that the specific metals—and not just the presence of particles in the cell—are driving the ROS generation. "It can be difficult separating the effect of the nanoparticle and the attached material," he says, "but this work clearly shows different effects between iron, titanium, cobalt, and manganese on particles that are similar in size, shape, and physical properties."
|
|
Although Stark's results add to the growing body of evidence that nanoparticle toxicity may be a concern, he emphasizes that they are still preliminary. He plans to extend his studies to examine the effect of ROS generation on the cells, using assays to assess genotoxicity and to compare nanoparticle behavior with other well-known toxic substances, although he says that additional work will be needed to translate in vitro results to living systems.
|
|
Nevertheless, Stark believes that assessments of potential nanoparticle toxicity should be conducted early in the development of new technologies. "Another part of my lab is doing application-oriented research," he explains, "and I don't want to be part of any application that is going to have to be corrected down the road."
|

