| Nov 26, 2010 | |
Revealing the secrets of chemical bath deposition |
|
| (Nanowerk News) X-ray absorption near-edge structure (XANES) spectroscopy is well known as a versatile and powerful technique for examining the microstructure of everything from crystalline solids to amorphous materials, even liquids. Its extreme sensitivity also makes it an ideal tool for probing the kinetics of various chemical reactions in situ. Experimenters utilizing the U.S. Department of Energy Office of Science's Advanced Photon Source at Argonne recently demonstrated a new wrinkle for XANES that has opened a window on a poorly-understood technique for deposition of materials. These insights will encourage the development of better-controlled and more precise chemical synthesis techniques for semiconductor and other nanomaterial applications, and are valuable as a demonstration of the extension of XANES spectroscopy into other realms of experimentation. | |
| While chemical bath deposition (CBD) is widely used in the laboratory and industry for the creation of thin films and nanostructures for semiconductors and photovoltaics, its actual molecular workings have remained something of a mystery. This has somewhat limited its utility, because precise tailoring of CBD products is not possible without a clear understanding and thus control of CBD mechanics. Scientists from Drexel University and the University of Notre Dame have obtained the first detailed look at how CBD operates at the molecular level, using XANES spectroscopy to witness in situ the formation of zinc oxide nanowires. The work was published in October 2010 in Chemistry of Materials ("In Situ X-ray Absorption Near-Edge Structure Spectroscopy of ZnO Nanowire Growth During Chemical Bath Deposition"). | |
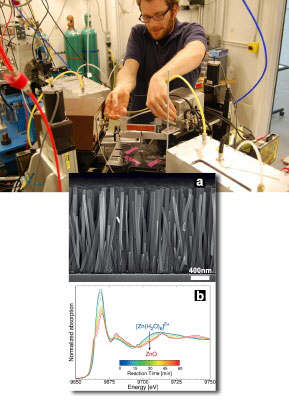 rexel University Ph.D. student Kevin McPeak prepares the microreactor for XANES spectroscopy at the MR-CAT 10-ID beamline. Inset: Scanning electron micrograph of ZnO nanowire array and in situ, time-resolved Zn K-edge XANES spectra of ZnO nanowire growth at 90°C showing transition from Zn(H2O)62+ to ZnO. CBD begins with a water solution with chemical precursors containing the components from which the desired film structure will be formed. But because the precursor chemical species tend to be very dilute within the solution, identifying and isolating them to monitor their activity during the deposition process has been a daunting challenge. "It's very difficult to find experimental techniques that will allow you to assess the different things that you need to measure," said principal investigator Jason Baxter of Drexel University. "This has led to some criticism of CBD for being too recipe-based, where it can be difficult to take one set of conditions and say what might happen elsewhere." XANES proved to be the ideal window into the CBD process. "It gives you very high sensitivity so you can measure species that are very dilute," Baxter said. "So we were able to look at CBD with a degree of accuracy that people could not achieve before." The researchers subjected a solution of zinc nitrate and HMTA (hexamethylenetetramine) to different temperatures and pressures inside a custom-built microreactor device to induce ZnO nanowire growth, observing the reactions with XANES spectroscopy at the Materials Research Collaborative Access Team (MR-CAT) beamline 10-ID at the Advanced Photon Source. Baxter points out a particular advantage of XANES for the current work: "It also has good enough time resolution that we could actually watch the reaction proceeding in time. Every minute we could take a new set of data and look at the kinetics of the reaction." One open question the researchers sought to address was the specific role of HMTA in the ZnO CBD process. Previous work had suggested that HMTA might break down into intermediate forms that provided the raw materials for the ZnO film, perhaps even binding to zinc ions in the solution, or that it might act simply as a pH buffer to facilitate the reactions. |
|
| This first in situ view afforded by the XANES technique demonstrated that HMTA decomposes slowly under heating, releasing hydroxide ions that react with zinc ions in the formation of ZnO. This slow release of hydroxides also has the effect of minimizing ZnO saturation and thus controlling the solution pH. | |
| "HMTA releases the hydroxide at the appropriate rate, just at the borderline where you're primarily growing zinc oxide on the substrate with minimal precipitation," says Baxter. | |
| The team observed the growth of ZnO nanowires from zinc nitrate and HMTA precursors at 90? C after two hours, with typical hexagonal cross-sections and diameters of 300-500 nm. | |
| They also employed principal component analysis (PCA) techniques to obtain quantitative data on the observed species during the CBD process. This showed that the ZnO nanowire growth occurred through direct crystallization from the precursor materials without any long-lived intermediates. The pH buffering provided by the HMTA helps to avoid overabundant precipitation of ZnO in the solution, allowing the controlled growth of the nanowire structures. | |
| These new insights into the mechanisms of CBD will encourage the development of better-controlled and more precise chemical synthesis techniques for semiconductor and other nanomaterial applications. | |
| The work is also valuable as a demonstration of the extension of XANES spectroscopy into other realms. | |
| "I think the more widely useful part of this paper is actually in the application of XANES spectroscopy to a new type of system," said Baxter. | |
| He and his team plan to extend their work to study other CBD chemistries and processes. "You can actually see what's happening as it is growing," he said. "It gives one a lot of information about the process. I think that's the exciting part." |
| Source: Argonne National Laboratory |
