| Dec 10, 2010 |
Wired up and ready to glow
|
|
(Nanowerk News) Thirty years ago, no one believed that elements other than carbon, nitrogen, and oxygen could form double bonds at room temperature. But the discovery of 'kinetic protection' ligands—large, bulky molecules that trap heavy atoms into multiply-bonded arrangements—forced a textbook rewrite. Researchers soon found that unsaturated bonds in newly synthesized substances such as disilenes, which have double silicon–silicon connections, generated chemical reactivity unlike any materials seen before.
|
|
Kohei Tamao and colleagues from the RIKEN Advanced Science Institute in Wako and Kyoto University have now discovered a way to make disilenes into thermally stable, light-emitting crystals by combining them with aromatic hydrocarbons ("Air-Stable, Room-Temperature Emissive Disilenes with -Extended Aromatic Groups"). The key to their approach is a protecting ligand known as 'Eind' that is rigid enough to lock carbon and silicon atoms into a wire-like network.
|
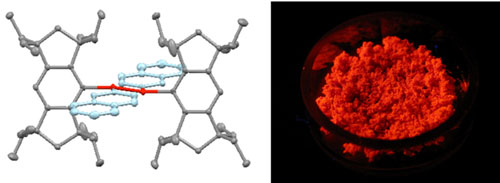 |
| Figure 1: The x-ray crystal structure of a new compound with silicon–silicon double bond and naphthyl groups (red/blue atoms) held in place by bulky 'Eind' ligands (grey atoms) (left). This stable material formed from this compound emits a fluorescent glow after ultraviolet irradiation (right).
|
|
Atoms make double bonds by sharing electrons through banana-shaped regions of space known as 'pi orbitals'. If enough pi orbitals exist in a molecule, they can overlap and create conjugated pathways that allow for easy movement of electrons—properties that make such materials extremely responsive to light.
|
|
Incorporating disilenes into organic conjugated systems could produce enhanced photo-activity, but positioning two types of double bonds in one geometric plane for maximum pi orbital overlap is difficult. In 2007, Tamao and his team solved this problem by developing Eind ligands to protect a disilene–benzene compound ("Coplanar Oligo(p-phenylenedisilenylene)s Based on the Octaethyl-Substituted s-Hydrindacenyl Groups"). Eind groups have a stiff framework of three fused hydrocarbon rings that encapsulate the pi network and force it into a planar geometry by stacking perpendicular to it, which enabled the disilene–benzene product to be isolated as a fluorescent orange solid.
|
|
In their latest work, the researchers investigated the effects of extended organic pi conjugation by adding naphthalene and fluorene groups—aromatic molecules nearly twice the size of benzene rings—to an Eind-protected disilene. X-ray analysis revealed that the resulting red crystals also had planar silicon–carbon conjugation, a structure that produced intense fluorescent light emissions after ultraviolet irradiation (Fig. 1). "We were excited to see such strong solid-state emissions even at room temperature," says Megumi Kobayashi, a co-author of the study.
|
|
Furthermore, these novel compounds showed unprecedented thermal stability, a critical requirement for future optical applications. "Usually, compounds having unsaturated silicon bonds are reactive and air-sensitive," says Tsukasa Matsuo, another co-author, "but our red disilenes are air-stable for almost one year." The team is currently working on improving the solubility of disilene–aromatic molecules to help develop longer and more light-sensitive double bonded materials.
|

