| Dec 13, 2010 |
An element of nobel-ity: Michigan Tech's carbon connection
|
|
(Nanowerk News) Who ever would have guessed that the business end of Dixon Ticonderoga No. 2 pencils would someday be the next big thing?
|
|
John Jaszczak, perhaps. He was not all that surprised that the 2010 Nobel Prize in Physics was presented to two Russian-born scientists who created atom-thin sheets of carbon, called graphene, made from graphite. Jaszczak, a professor of physics and adjunct curator of the Seaman Mineral Museum, is a longtime fan of the mineral and was familiar with their prize-winning work. In fact, he supplied the researchers, Andre Geim and Konstantin Novoselov of the University of Manchester, with graphite crystals to use in their experiments. And, he appears as a coauthor on one of their papers, "Giant Intrinsic Carrier Mobilities in Graphene and Its Bilayer," published in Physical Review Letters.
|
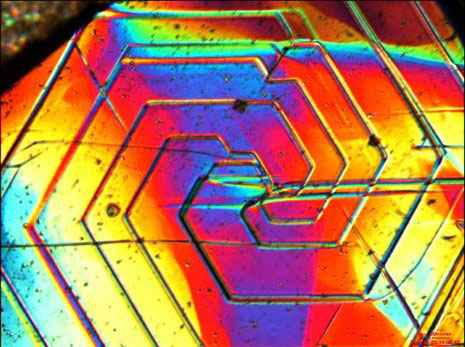 |
| Growth spirals on graphite crystals. Colors are generated by the optical microscope to reveal the features.
|
|
Jaszczak's graphite is not your garden-variety pencil lead. For about ten years, he has been supplying scientists with rare forms of the mineral, especially high-quality single crystals, isolated from rocks from select localities ranging from New York and California to Tanzania.
|
|
Because of its potentially more-perfect crystalline structure compared to synthetic graphite, Geim and Novoselov likely thought that Jaszczak's natural graphite might help them produce even better quality graphene. Just one atom thick, it is the world's thinnest and strongest nanomaterial, almost transparent and able to conduct electricity and heat. "When they make graphene from most graphite, the resulting graphene flakes are very small, typically only a few microns across," said Jaszczak. "Using our graphite crystals, which are well-ordered on a scale from 500 microns to several millimeters across, scientists hope to be able to obtain graphene that is not only of larger dimensions but of a higher degree of order. Dr. Geim was hopeful that the higher degree of order would lead to even more remarkable electronic properties for resulting graphene."
|
|
Jaszczak gave up the graphite sales business in 2008, handing it over to the student-run Nanotech Innovations Enterprise for one of their projects. Since then, the team has established Naturally Graphite, "supplying high-quality, natural graphite crystals for research and education" from their website, graphitecrystals.com. Among their customers are NIST, the Sandia and Brookhaven national labs, Georgia Tech and MIT, who use the crystals for graphene research as well as substrates for scanning tunneling microscopy. With their profits, the students support the work of the Enterprise, including educational programs for high school students and teachers.
|
|
No Michigan Tech graphite was used in the making of the Nobel Prize-winning graphene, Jaszczak sighs. Nevertheless, students in the Nanotechnology Enterprise are more than satisfied with their business outcomes. "We are supplying scientists with materials that otherwise would be unavailable," said Jaszczak. "We also enjoy demonstrating our scanning tunneling microscope during numerous outreach events. We use it to show the atomic-scale network of carbon atoms on a graphite crystal and measure the carbon-carbon bond distance. The kids think it's amazing. So do I!"
|

