| Dec 17, 2010 |
Novel injectable hydrogel with tunable stiffness for tissue repair and regeneration
|
|
(Nanowerk News) Scientists at the Institute of Bioengineering and Nanotechnology (IBN), the world's first bioengineering and nanotechnology research institute, have developed the first injectable hydrogel system with variable stiffness that can control cell proliferation and differentiation in a two-dimensional (2D) and three-dimensional (3D) cell culture environment. This unique hydrogel invention has important tissue engineering applications, especially in the treatment of neurological disorders, brain and muscle injuries ("The role of stiffness of gelatin-hydroxyphenylpropionic acid hydrogels formed by enzyme-mediated crosslinking on the differentiation of human mesenchymal stem cell").
|
|
Human mesenchymal stem cells (hMSCs) are commonly used in cell therapies because they are easy to isolate and handle. hMSCs can differentiate into multiple cell types, such as osteoblasts (bone cells), neurons (nerve cells) and cardiac myocytes (heart muscle cells) via cell culture. The use of hydrogels as scaffolds for the cultivation of hMSCs is attractive because the former's high water content matrix provides high permeability for oxygen, nutrients and other water-soluble metabolites. This quality makes them an excellent environment for cell growth and tissue regeneration. In addition, hydrogels can administer cells to the precise locations in the body for tissue repair and regeneration.
|
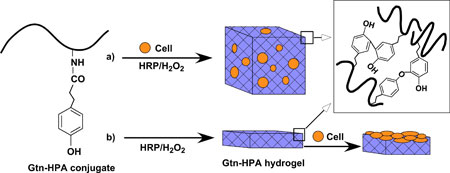 |
| Formation of Gtn–HPA hydrogels by enzyme-catalyzed oxidation for (a) 3D and (b) 2D cell growth/differentiation.
|
|
Hydrogel stiffness directly affects cell proliferation and differentiation, and a major challenge of existing injectable hydrogel systems lies in controlling the hydrogel's stiffness without affecting its gelation rate. Hydrogels are formed via a crosslinked polymer network and control over the gelation rate is presently limited to varying the polymer precursor solution, which changes the stiffness but is undesirable for controlling cell growth and differentiation. This means that when hydrogels with low stiffness are formed from a low concentration of polymer precursor, the gelation rate will be correspondingly slower. For an injectable hydrogel system, it is important for the hydrogel to be formed rapidly after injection, to prevent the unwanted diffusion of the gel precursors and cells to the surrounding tissues ("Injectable Biodegradable Hydrogels with Tunable Mechanical Properties for the Stimulation of Neurogenesic Differentiation of Human Mesenchymal Stem Cells in 3D Culture").
|
|
To overcome this limitation, IBN has developed a novel injectable hydrogel system composed of biodegradable gelatin–hydroxyphenylpropionic acid (Gtn–HPA) conjugates that provides independent control over the stiffness and gelation rate of the hydrogel. The Gtn–HPA hydrogels were formed via an enzyme-mediated oxidation reaction with hydrogen peroxide (H2O2) and horseradish peroxidase (HRP), which determined the stiffness and gelation rate, respectively. By modifying the H2O2 concentration, the mechanical strength of the Gtn–HPA hydrogels can be altered without changing the polymer precursor solution and affecting its gelation rate over a wide range of stiffness. In the 2D and 3D culture setting, IBN researchers found that the proliferation of hMSCs inside the hydrogel increased with a decrease in stiffness and the neurogenesic differentiation was also enhanced when the cells were cultured in hydrogels with lower stiffness.
|
|
Additionally, IBN's injectable hydrogel system is synthesized without toxic chemicals, allowing bioactive molecules, such as therapeutic proteins, growth factors and cells to be encapsulated in the hydrogels without being damaged. This biodegradable system allows the hydrogels to be formed without causing any inflammatory response in the body.
|
|
"With its readily tunable mechanical properties and excellent performance as a cell growth support, our injectable Gtn-HPA hydrogel system has demonstrated its suitability as a tissue engineering scaffold. Our research findings show the importance of a suitable material system and the influence of physical parameters in addition to biological and chemical parameters for effective tissue repair and regeneration. Our hydrogels' excellent cell adhesion and their optical transparency also make them suitable for tissue engineering applications," said Dr Motoichi Kurisawa, IBN Team Leader and Principal Research Scientist, who led this research effort.
|
|
"Developing new approaches to medical treatments using next-generation materials is a key research thrust in IBN. This latest biomaterial innovation from our scientists offers a promising breakthrough, particularly for the advancement of stem cell-based in vivo therapies. We are now able to deliver a simple and biocompatible system to advance tissue engineering and drug delivery applications," added Professor Jackie Y. Ying, IBN Executive Director.
|
|
About the Institute of Bioengineering and Nanotechnology
|
|
The Institute of Bioengineering and Nanotechnology (IBN) was established in 2003 and is spearheaded by its Executive Director, Professor Jackie Yi-Ru Ying, who has been on the Massachusetts Institute of Technology's Chemical Engineering faculty since 1992, and was among the youngest to be promoted to Professor in 2001.
|
|
In 2008, Professor Ying was recognized as one of "One Hundred Engineers of the Modern Era" by the American Institute of Chemical Engineers for her groundbreaking work on nanostructured systems, nanoporous materials and host matrices for quantum dots and wires.
|
|
Under her direction, IBN conducts research at the cutting-edge of bioengineering and nanotechnology. Its programs are geared towards linking multiple disciplines across all fields in engineering, science and medicine to produce research breakthroughs that will improve healthcare and our quality of life.
|

