| Jan 14, 2011 |
Simulations explain the peculiar nature of molecular hydrogen vibration under high pressure
|
|
(Nanowerk News) Most of our Universe consists of hydrogen atoms, which are often found under extraordinarily high pressure as high as tens of millions of times the atmospheric pressure of Earth. Understanding the exotic physics of such a high-pressure regime will contribute to our understanding of planet formation, hydrogen storage, room temperature superconductivity and other fields, explains Toshiaki Iitaka from the RIKEN Advanced Science Institute in Wako.
|
|
Iitaka, along with colleagues from the Institute of High Performance Computing in Singapore and the University of Saskatchewan in Canada, recently uncovered the physical basis underlying a newly discovered behavior of hydrogen molecules under high pressure ("Pressure-Induced Intermolecular Interactions in Crystalline Silane-Hydrogen").
|
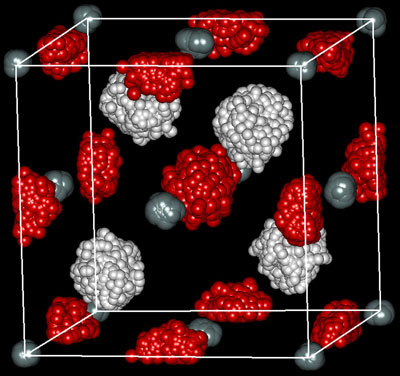 |
| Figure 1: Traces of the positions of silane and hydrogen molecules over time at 32 GPa, obtained from molecular dynamics calculations. Hydrogen atoms at tetrahedral (white) and octahedral (red) sites are shown. Silicon atoms at so-called 'face-centered cubic' sites are shown by gray spheres.
|
|
This behavior was observed in a complex of hydrogen molecules, and hydrogen bound to silicon, which is called silane. Silane's hydrogen atoms are under so-called 'chemical compression' by virtue of their being part of a chemical bond. In 2009, physicists found that the vibrational frequency of hydrogen molecules in silane–hydrogen complexes fell as the applied pressure rose. This anti-correlation was the opposite of previous observations of high-pressure hydrogen.
|
|
Iitaka and colleagues modeled the system using molecular dynamics simulations. They first optimized the relative arrangement of hydrogen and silane molecules inside a unit cell, finding that the hydrogen molecules tend to sit at octahedral and tetrahedral sites (Fig. 1). They then computed the vibrational frequencies of the hydrogen molecules, and found two groups of vibrational modes, one at high energy and one at low energy.
|
|
The frequencies of the lower-energy group decreased monotonically as applied pressure increased. However, the frequencies of the higher-energy group increased with pressure until about 20.1 giga Pascals (GPa), after which they fell. This reproduced the experimentally observed anti-correlation between vibrational frequency and applied pressure, indicating that the simulation was accurate.
|
|
The simulations also revealed that this rise and fall in frequencies resulted from interactions between hydrogen and silane molecules. These interactions resulted from the overlap between the filled electron orbitals of one molecule and the empty orbitals of the other molecule. This overlap stabilizes the system, and its strength depends on the distance between the molecules. This distance, in turn, depends on the applied pressure.
|
|
The simulation results are another glimpse into the exotic physics that underpins the high-pressure regime, according to Iitaka. "We have shown that there is much more interesting new physics and chemistry to be explored in the world of high pressure."
|

