| Mar 09, 2011 |
Synthetic biology researchers develop novel kind of fluorescent protein
|
|
(Nanowerk News) Since the middle of the 1990s a bright green fluorescent protein has been used in research laboratories worldwide. Protein designers at Technische Universitaet Muenchen (TUM) in Weihenstephan have now taken the existing fluorescent protein a step further: They have managed to incorporate a synthetic amino acid into the natural protein and thus to create a new kind of chimeric fluorescent bio-molecule by means of synthetic biology. By exploiting a special physical effect, the fluorescent protein glows in turquoise when excited with ultraviolet light and displays up to now unmatched properties.
|
|
The scientists present their results in the renowned international Journal of the American Chemical Society ("Biosynthesis of a Fluorescent Protein with Extreme Pseudo-Stokes Shift by Introducing a Genetically Encoded Non-Natural Amino Acid outside the Fluorophore").
|
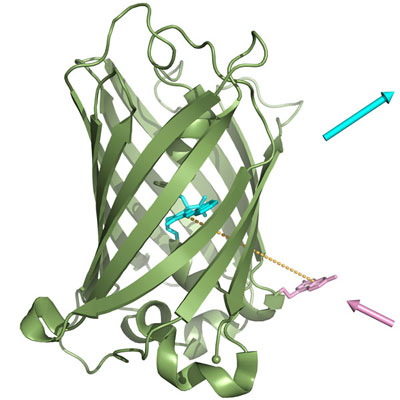 |
| Interplay of two dyes in the biosynthetic fluorescent protein. (Graphics: A. Skerra/TUM)
|
|
Proteins are the most important functional biomolecules in nature with numerous applications in life science research, biotechnology and medicine. So how can they be modified in the most effective way to attain certain desired properties? In the past, the modifications were usually carried out either chemically or via genetic engineering.
|
|
The team of Professor Arne Skerra from the TUM Chair of Biological Chemistry has now developed a more elegant combined solution: By extending the otherwise universal genetic code, the scientists are able to coerce bacterial cells to produce tailored proteins with synthetic functional groups. To put their idea to the test, they set out to crack a particularly hard nut: The scientists wanted to incorporate a non-natural amino acid at a specific site into a widely used natural protein.
|
|
In bioresearch this protein is commonly known as "GFP" (= green fluorescent protein). It emits a bright green glow and stems originally from a jellyfish that uses the protein to make itself visible in the darkness of the deep sea. The team chose a pale lavender coumarin pigment, serving as side chain of a non-natural amino acid, as the synthetic group. The scientists "fed" this artificial amino acid to a laboratory culture of Escherichia coli bacteria – the microorganism workhorses of genetic engineering, whose natural siblings are also found in the human intestine. Since the team had transferred the modified genetic blueprints for the GFP to the bacteria – including the necessary biosynthesis machinery – it incorporated the coumarin amino acid at a very specific site into the fluorescent protein.
|
|
This spot in the GFP was carefully chosen, explains Professor Skerra: "We positioned the synthetic amino acid at a very close distance from the fluorescence center of the natural protein." The scientists employed the principle of the so-called Förster resonance energy transfer, or FRET for short. Under favorable conditions, this process of physical energy transfer, named after the German physical chemist Theodor Förster, allows energy to be conveyed from one stimulated pigment to another in a radiation-less manner.
|
|
It was precisely this FRET effect that the scientists implemented very elegantly in the new fluorescent protein. They defined the distance between the imported chemical pigment and the biological blue-green (cyan, to be more precise) pigment of the jellyfish protein in such a way that the interplay between the two dyes resulted in a completely novel kind of fluorescent chimeric biomolecule. Because of the extreme proximity of the two luminescent groups the pale lavender of the synthetic amino acid can no longer be detected; instead, the typical blue-green color of the fluorescent protein dominates.
|
|
"What is special here, and different from the natural GFP, is that, thanks to the synthetically incorporated amino acid, the fluorescence can be excited with a commercially available black-light lamp in place of an expensive dedicated LASER apparatus," explains Sebastian Kuhn, who conducted these groundbreaking experiments as part of his doctoral thesis.
|
|
According to Skerra, the design principle of the novel bio-molecule, which is characterized by a particularly large and hard to achieve wavelength difference between excitation and emitted light, should open numerous interesting applications: "We have now demonstrated that the technology works. Our strategy will enable the preparation of customized fluorescent proteins in various colors for manifold future purposes."
|
|
This research project was financially supported by the German Research Foundation (DFG) as part of the Excellence Cluster "Munich Center for Integrated Protein Science"
|

