| Apr 27, 2011 |
An overlooked detail may invalidate the results of some prior experiments with nanoparticles
|
|
(Nanowerk News) As any bench scientist will tell you, experimental design can be the very devil.
|
|
Try as one might, it can be difficult to recognize, much less eliminate, the many extraneous factors that might bias an experiment. And unrecognized confounding factors can invalidate years of work.
|
|
So scientists worry. Recently Washington University in St. Louis scientist Younan Xia started to worry about in vitro experiments his lab was doing to study the uptake of nanoparticles by living cells.
|
|
In the laboratory, the cells were always plated on the bottom of a dish and the culture medium containing nanoparticles added from the top.
|
|
"People assumed that if they prepared a suspension, the suspension was going to have the same concentration everywhere, including at the surface of the cells, " says Xia, PhD, the James M. McKelvey Professor in the Department of Biomedical Engineering, says.
|
|
A battery of experiments in Xia's lab with both upright and inverted setups showed that nanoparticles above certain sizes and weights will settle out. So concentrations of the nanoparticles near the cell surfaces are different from those in the bulk solution and cellular uptake rates are higher.
|
|
This issue is important because scientists are actively investigating the use of nanoparticles as vehicles for the delivery of drugs or genes to cells.
|
|
For these applications, calculations of the dose the particles actually convey to cells are crucially important.
|
|
As the scientists conclude in the Nature Nanotechnology article describing the experiments, "Studies on the cellular uptake of nanoparticles that have been conducted with cells in the upright configuration may have given rise to erroneous and misleading data."
|
|
The swan's neck effect
|
|
Spontaneous generation—the idea that living organisms such as maggots arise from dead matter—persisted into the 19th century because people who tried to test it had trouble with experimental design.
|
|
They were particularly vexed by air. Should air be included or excluded from the flask holding a nourishing broth? Air might be necessary for spontaneous generation, as it is for combustion, or it might waft in microorganisms whose presence would invalidate a positive result.
|
|
So spontaneous generation, an idea familiar to Aristotle, wasn't definitively disproved until the 19th century French scientist Louis Pasteur came up with an experimental design that separated the two roles of air. He boiled broth in a flask, then heated the neck of the flask and bent it into a swan's neck.
|
|
Air could enter the flask, but microorgansisms in the air settled out in the bend in the neck.
|
|
The broth stayed clear, definitively proving that life does not arise spontaneously and comes only from life.
|
|
Topsies and Turveys
|
|
Until now nanoparticles were assumed to be well-dispersed in the culture medium because they are small enough to be easily lofted by Brownian motion, the random motion of the molecules in the medium.
|
|
Therefore scientists felt they could safely assume that the concentration of nanoparticles in the fluid next to the cells, which drives cellular uptake, was the same as the initial concentration of nanoparticles in the medium.
|
|
"We started to wonder, however, because our nanoparticles are made of gold," Xia says. "Gold is nontoxic but it is also very heavy, so it was conceivable relatively large nanoparticles might settle."
|
|
Since it is impossible to measure the exact concentration of gold nanoparticles at the surface of a cell, Xia and coworkers designed a simple experiment to clearly see the difference in concentration caused by sedimentation.
|
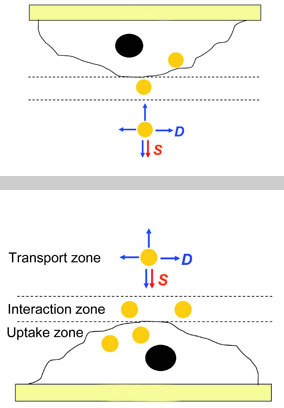 |
| The experiments in Xia's lab compared the usual experimental setup (bottom) to an inverted setup (top). Nanoparticle uptake in the two setups differs only if the ratio of the forces driving sedimentation (S) to those driving diffusion (D) are different. In the situation shown here the upright cells have taken up more nanoparticles than the inverted ones.
|
|
Xia's lab tested gold nanospheres of three sizes, nanocages of two edge lengths, and nanorods, some with surface coatings that picked up serum proteins in solution and others coated with a chemical that acts as an antifouling agent.
|
|
After the cells were incubated in the nanoparticle-bearing medium, the concentration of the nanoparticles was measured spectroscopically and the number of particles each cell had taken up was then calculated.
|
|
In the literature, Xia says, there are reports that the cellular uptake of nanoparticles depends on the nanoparticles' size, shape and surface coating.
|
|
His lab's experiments showed that these characteristics are secondary, relevant only insofar as they affect the sedimentation and diffusion velocities of the nanoparticles.
|
|
For small, light particles, there was no disparity between the cells in the upright and the inverted configurations. In the case of larger, heavier particles, however, sedimentation dominated, and the upright cells took in more nanoparticles than the inverted cells.
|
|
"All earlier work may need to be re-evaluated to account for the effects of sedimentation on nanoparticle dosimetry," the authors conclude.
|
|
"It's no different from medicines that have to be shaken to suspend a powder in a water. If you don't shake the bottle," Xia says, "you end up under- or overdosing yourself."
|

