| May 01, 2011 |
Cell signals via membrane nanotubes
|
|
(Nanowerk News) For nearly ten years, researchers have known that cells can "grow" ultra-thin tubes named tunnelling nanotubes (TNTs) between one another. These nanotubes – the length of two to three cells and just 1/500th the thickness of a human hair – are connections that develop between nearly all cell types to form a communication channel different from any previously known mechanisms.
|
|
In 2010, Dr. Xiang Wang and Professor Hans-Hermann Gerdes – colleagues at the University of Bergen's Department of Biomedicine – discovered that electrical signals were being passed through nanotubes from one cell to another at high speed (roughly 1-2 m/sec). Their research receives funding under the Research Council's large-scale research programme Nanotechnology and New Materials (NANOMAT).
|
 |
| Dr. Xiang Wang and Professor Hans-Hermann Gerdes of the University of Bergen have discovered a previously unknown communication system between cells. (Photo: UiB)
|
|
The breakthrough
|
|
In their key experiment, Dr Wang used fluorescent dye that changes in intensity as the electric potential of the cell membrane changes. When two cells connected by forming a nanotube, he poked into one of them with a microinjection needle to depolarise that cell's membrane potential. This caused the fluorescent indicator on the cell membrane to light up like a firework, and it was soon followed by a similar light display in the cell on the other end of the nanotube.
|
|
The breakthrough discovery began with an experiment demonstrating intercellular transmission of electrical signals via nanotubes in 2007. The researchers then carried out similar trials with a number of other cell types, observing similar occurrences.
|
|
"We confirmed that this is a common phenomenon between cells," explains Professor Gerdes. "Still, this characteristic is not in every cell type."
|
|
The experiment was replicated a number of times to obtain statistically reliable data. The electrophysiology group at the University of Bergen took precise conductivity measurements of the cell systems to determine the strength of the electrical coupling. In autumn 2010 the results were published in Proceedings of the National Academy of Sciences (PNAS) and were highlighted as top news in Nature News.
|
|
Intercellular nanotubes are far from permanent. Most of them last only a few minutes. This means the researchers cannot predict where and when the cells will form nanotube connections.
|
|
"It is truly painstaking work," says Professor Gerdes. "You may sit there examining cells for hours through a microscope without seeing a single tube. If you are lucky, however, you catch sight of a nanotube being created and can film the event."
|
|
To raise the likelihood of finding nanotubes, the researchers developed a micro-matrix consisting of thousands of points and bridges on a plate surface. Smaller than a postage stamp, the plate is covered by a nano-structured material to which the cells adhere. The researchers place one cell onto each point and hope that nanotubes will form along the bridges between the points. The camera is focused on these bridges.
|
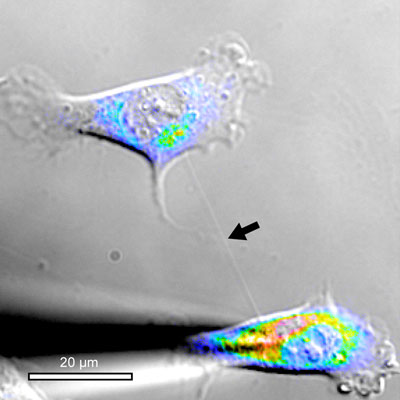 |
| Here a cell has coupled with another cell by growing a long nanotube which enables it to exchange electrical signals. (Photo: UiB)
|
|
Once the nanotubes have been established, the researchers manipulate the cells at specified times; meanwhile the microscope is programmed to photograph, say, 50 preselected points every five minutes. The team can thus obtain data about many nanotube connections in a short time.
|
|
How do cells do this?
|
|
Dr. Wang quickly discovered that the mere presence of a nanotube was not sufficient to transmit an electrical signal. There had to be another mechanism involved as well.
|
|
Many cells form tiny membrane pores with each other called gap junctions, which are made up of ring-shaped proteins. Back in the 1960s it was discovered that directly adjacent cells could exchange electrical impulses through these gap junctions. What Dr Wang found was that one end of the nanotube was always connected to cells by a gap junctions before it transmitted its electrical impulses.
|
|
He also found that in some coupled cells voltage-gated calcium channels were involved in the forwarding of the incoming signals. When the electrical signal being sent through the nanotube reaches the membrane of the receiving cell, the membrane surface is depolarised, opening the calcium channel and allowing calcium – a vital ion in cell signalling – to enter.
|
|
"In other words," explains Professor Gerdes, "there are two components: a nanotube and a gap junction. The nanotube grows out from one cell and connects to the other cell through a gap junction. Only then can the two cells be coupled electrically."
|
|
Controls embryonic cells?
|
|
Now the scientists are seeking answers as to why the cells send signals to each other in this way.
|
|
"It's quite possible that the discovery of nanotubes will give us new insight into intercellular communication," asserts Professor Gerdes. "The process could explain how cells are coordinated during embryo growth. In that phase cells travel long distances – yet they demonstrate a kind of collective behaviour, and move together like a flock of birds can."
|
|
Nanotubes may also be a factor in explaining cell movement associated with wound healing, since cells move toward a wound in order to close it. We already know that electrical signals are somehow involved in this process; scientists can only speculate as to whether nanotubes are involved here as well, stresses Professor Gerdes.
|
|
Perhaps brain cells, too?
|
|
In terms of electronic signal processing, the human brain surpasses all other organs. If this same signalling mechanism proves to be present in human brain cells, it could add a new dimension to understanding how the brain functions. Communication channels involving synapses and dendrites that are already identified differ widely from nanotubes.
|
|
The Bergen-based neuroscientists see this research as an opportunity to formulate better explanations for phenomena related to consciousness and electrical connections in the brain. In the project "Cell-to-cell communication: Mechanism of tunnelling nanotube formation and function", they are now studying precisely how nanotube mechanisms function in brain cells.
|
|
Professor Gerdes is currently conducting research at the European Molecular Biology Laboratory in Heidelberg. By studying the electrical connections in vivo he hopes to figure out how the mechanisms work in live subjects. The results could enhance understanding of diseases that occur when cell mechanisms fail to function properly.
|


