| May 09, 2011 |
Low-cost, efficient catalyst boosts hydrogen fuel technology
|
|
(Nanowerk News) A collaboration of scientists from DTU, SLAC National Accelerator Laboratory and Stanford University recently published their results in Nature Materials ("Bioinspired molecular co-catalysts bonded to a silicon photocathode for solar hydrogen evolution"). The discovery is an important development in the worldwide effort to mimic the way plants make fuel from sunlight, a key step in creating a green energy economy.
|
|
Hydrogen is an energy dense and clean fuel, which upon combustion releases only water. Today, most hydrogen is produced from natural gas which results in large CO2-emissions. An alternative, clean method is to make hydrogen fuel from sunlight and water. The process is called photo-electrochemical, or PEC, water splitting. When sun hits the PEC cell, the solar energy is absorbed and used for splitting water molecules into its components, hydrogen and oxygen.
|
|
Progress has so far been halted in part by the lack of cheap catalysts that can speed up the generation of hydrogen and oxygen. A vital part of the American-Danish effort was combining theory and advanced computation with synthesis and testing to the process of identifying new catalysts. This is a new development in a field that has historically relied on trial and error. "If we can find new ways of rationally designing catalysts, we can speed up the development of new catalytic materials enormously," CASE Leader Jens Nørskov said, who is head of the research group at SLAC National Accelerator Laboratory and Stanford University.
|
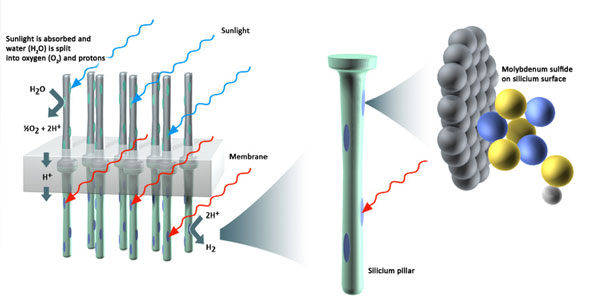 |
| When sunlight strikes the silicium pillars, the energy is absorbed and used for splitting water into oxygen (O2) and free protons which are then turned into hydrogen gas (H2). (Adapted from Lewis, N. S. & Nocera, D. G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 103, 15729-15735 (2006) and Grey, H. B. Powering the planet with solar fuel. Nature Chem. 1, 7 (2009))
|
|
The team first tackled the hydrogen half of the problem. The DTU researchers created a device to harvest the energy from part of the solar spectrum and used it to power the conversion of single hydrogen ions into hydrogen gas. However, the process requires a catalyst to facilitate the reaction. Platinum is already known as an efficient catalyst, but platinum is too rare and too expensive for widespread use. So the collaborators turned to nature for inspiration.
|
|
They investigated hydrogen producing enzymes from certain organisms, using a theoretical approach Nørskov's group has been developing to describe the behavior of catalysts.
|
|
"We did the calculations," Nørskov explained, "and found out why these enzymes work as well as they do." These studies led them to related compounds, which eventually took them to molybdenum sulfide. "Molybdenum is an inexpensive solution" for catalyzing hydrogen production, said Professor Ib Chorkendorff from the Center for Individual Nanoparticle Functionality (CINF) who was leading the molybdenum sulfide experiments.
|
|
The team also optimized parts of the device, introducing a "chemical solar cell" designed to capture as much solar energy as possible. The experimental researchers at DTU designed light absorbers that consist of silicon arranged in closely packed pillars, and dotted the pillars with tiny clusters of the molybdenum sulfide. When they exposed the pillars to light, hydrogen gas bubbled up—as quickly as if they'd used costly platinum.
|
|
The hydrogen gas-generating device is only half of a full photo-electrochemical cell. The other half of the PEC would generate oxygen gas from the water—though hydrogen gas is the goal, without the simultaneous generation of oxygen, the whole PEC cell shuts down. Many groups—including Chorkendorff, Dahl and Nørskov and their colleagues—are working on finding catalysts and sunlight absorbers to do this well. "This is the most difficult half of the problem, and we are attacking this in the same way as we attacked the hydrogen side," CASE Leader Søren Dahl said.
|
|
Nørskov looks forward to solving that problem as well. "A sustainable energy choice that no one can afford is not sustainable at all," he said. "I hope this approach will enable us to choose a truly sustainable fuel."
|

