| May 23, 2011 |
Nanometer-scale layers between materials have both solid and liquid characteristics
|
|
(Nanowerk News) Researchers at the Technion have discovered the nature of nanometer-thick layers between different materials and found that they have both solid and liquid properties. By doing so, the researchers made a crucial addition to Gibbs' theory which describes the fundamental aspects of the thermodynamics of interfaces.
|
|
The recently published paper in Science experimentally demonstrates that the formation of a very thin layer (of the order of one nanometer in thickness) at interfaces reduces the interface energy, and promotes adhesion and interface stability. The thin layer is not a conventional state of matter, in that it is neither a solid nor a liquid, but rather something in between.
|
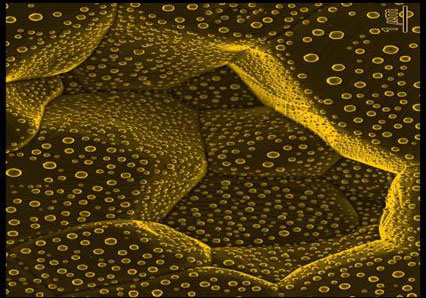 |
| Gold crystals equilibrated on a polycrystalline sapphire (aluminum oxide) surface.
|
|
The results could enable scientists to improve the resilience of the bond between ceramic materials and metals, two types of materials that "do not like" to come into contact. The many real-world applications include cutting tools for metal-working; composites for brake pads; the joins between metal conducting wires and chips in computers; and the application of protective ceramic coatings on jet engine blades.
|
|
"Until now, no one had been able to understand why these thin layers exist, or if they were a temporary or an equilibrium state," explains Prof. Wayne D. Kaplan, Dean of the Department of Materials Engineering at the Technion. "While their existence at interfaces between ceramic crystals and on the surface of ice was known, there was an ongoing debate about the cause of this phenomenon and its properties."
|
|
Through a long series of experiments, Dr. Mor Baram proved that a thin layer exists at the interface between metals and ceramic materials, which reduces the interface energy. The research was Dr. Baram's doctoral work, and was carried out under the supervision of Prof. Kaplan in cooperation with Dr. Dominique Chatain of CNRS in France.
|
|
"This phenomenon enables us to ice-skate, reduces the mechanical properties of ceramic materials at high temperatures, affects the morphology of crystals in modern polycrystalline engineering materials, and contributes to the stability of modern micro-electronic devices," says Prof. Kaplan.
|
|
The team conducted novel experiments at the Technion using the new "Titan" electron microscope and a focused ion beam (FIB). This included plating sapphire with a thin (0.6 micron thick) film of gold (for comparison, a single strand of hair is 80-100 microns thick), and heating the samples until they reached equilibrium (i.e. until the gold film broke-up into billions of tiny gold crystals on the sapphire). The researchers also included a source of elements on the sapphire known to play a role in forming the layer between different materials (in this case, silicon and calcium). As the samples reached equilibrium, the calcium and silicon moved to the interface between the gold and sapphire, and a thin (0.0012 microns, or 1.2 nanometers thick) layer was created.
|
|
The researchers were then able to successfully measure the energy stored between the gold and sapphire in the presence of the thin layer. By doing so, they proved that its presence decreases the energy of the interface, and therefore improves its stability.
|

