| May 27, 2011 |
Tiny probes for living cells
|
|
(Nanowerk News) Living cells are virtuosos of chemistry. At any one time, countless chemical reactions are taking place within each cell. For researchers trying to understand how cells function, unraveling this complex chemistry is an ongoing challenge. The process, however, could soon become a little more straightforward. Mikiko Sodeoka and colleagues at the RIKEN Advanced Science Institute at Wako, in collaboration with a team led by Katsumasa Fujita and Satoshi Kawata at Osaka University, have demonstrated how to tag molecules in a way that promises to be much more versatile than current methods ("Imaging of EdU, an Alkyne-Tagged Cell Proliferation Probe, by Raman Microscopy").
|
|
Traditionally, researchers looking to study the role of a particular small molecule within a cell have tagged it with a fluorescent marker. Using a fluorescence microscope, the tagged substance can be followed as it moves around the cell. However, fluorescent tags are bulky, and so can disrupt the molecule's normal cellular interactions. To get around this problem, molecules can sometimes be tagged after reaching their destination within the cell, but this technique only works in a limited number of cases.
|
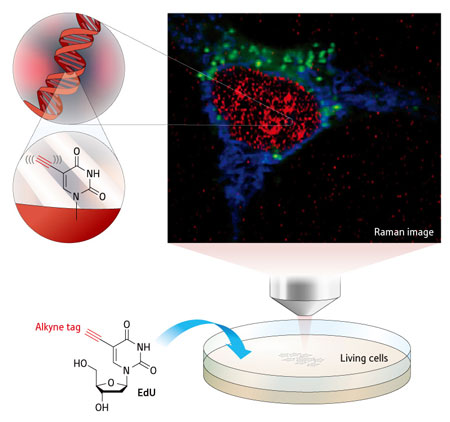 |
| Figure 1: Raman imaging reveals that a molecule known as EdU, colored red in this image, gathers in the cell nucleus. Cytochrome c and lipid are colored in blue and green respectively. (© 2011 American Chemical Society)
|
|
Sodeoka and her team have now shown that a simple chemical substituent called an alkyne, which consists of just two carbon atoms joined together by a triple bond, can replace the bulky fluorescent tag. Their imaging technique relies on the fact that alkynes scatter a particular wavelength of light when irradiated with a laser—a process known as Raman scattering—which can be detected using a Raman microscope. No other cellular components scatter light at this wavelength, giving a clear picture of the molecule within the cell.
|
|
To demonstrate the potential of their technique, the researchers used an alkyne-tagged component of DNA known as EdU. They then used a Raman microscope developed by Fujita and his team to follow a group of replicating cells as they incorporated EdU into their DNA (Fig. 1). The technique took some work to optimize, says Sodeoka. "We were very happy when we could finally see the time-series pictures of the incorporation of EdU into DNA."
|
|
The EdU experiment is just a proof of principle, Sodeoka adds. "At this point, the sensitivity of the alkyne tag using Raman microscopy is lower than fluorescent imaging," she says. To improve the sensitivity, the team is working to optimize the attachment of the alkyne tag, and also to improve the Raman microscope itself. "If the sensitivity problem is solved, Raman imaging using alkynes as a small tag could become a powerful tool," she concludes.
|

