| Jun 13, 2011 |
Graphene: From spheres to perfect dots
|
|
(Nanowerk News) An electron trapped in a space of just a few nanometers across behaves very differently to one that is free. Structures that confine electrons in all three dimensions can produce some useful optical and electronic effects. Known as quantum dots, such structures are being widely investigated for use in new types of optical and electronics technologies, but because they are so small it is difficult to fabricate quantum dots reproducibly in terms of shape and size. Researchers from the National University of Singapore (NUS) and A*STAR have now developed a technique that enables graphene quantum dots of a known size to be created repeatedly and quickly ("Transforming C60 molecules into graphene quantum dots").
|
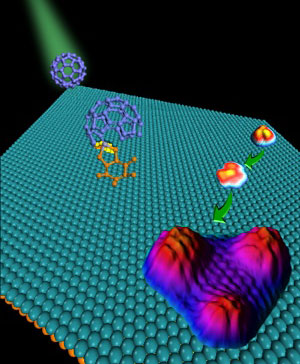 |
| Schematic illustration of the formation of graphene quantum dots from fullerenes using a ruthenium catalyst.
|
|
Past attempts to create graphene quantum dots have involved the use of hydrocarbon molecules as the starting point. Instead, the researchers started with a well-known spherical carbon structure called a C60 fullerene, which consists of 60 carbon atoms that are linked together in a soccer ball-like arrangement to form a sphere. The advantage of using fullerenes as the precursor is that the diffusion of carbon atoms can be better controlled since the size and shape of all fullerenes of the same type are identical.
|
|
Fullerenes can be opened up at high temperature using ruthenium as a catalyst. Previous experiments involving this catalyst, however, resulted in featureless clusters or graphene sheets rather than quantum dots. "Graphene in the form of extended sheets does not possess an energy gap and so cannot be used in transistors, whereas graphene quantum dots can have a size-tunable band gap," explains Kian Ping Loh from the NUS Graphene Research Centre.
|
|
The researchers succeeded in breaking down the fullerene to form individual graphene quantum dots by performing the decomposition using a sparser coverage of fullerenes on the catalytic ruthenium surface. This gave the fullerenes room enough to prevent carbon atoms from diffusing from one fullerene to the next. Upon heating the substrate to high temperature, the fullerenes decomposed into carbon clusters that subsequently diffused, aggregated and recrystallized into quantum dots (see image). Just a minute or two of heating at 725 K resulted in triangular graphene quantum dots, while further annealing at 825 K gave perfectly hexagonal dots just 5 nm in size.
|

