| Jun 20, 2011 |
Measuring the forces of interactions between quantum dots and living cells
|
|
(Nanowerk News) Ultrasmall particles are poised to become central to biomedical applications such as drug delivery and photodynamic therapies. Semiconductor nanoparticles called quantum dots (QDs) are particularly promising for biological imaging, having size-tunable light emission and excellent photostability. The development of such clinical applications, however, hinges on understanding how such nanoparticles interact with and penetrate living cells. A research team led by Hongda Wang from the Chinese Academy of Sciences in Changchun, China, has now developed a method to measure these interaction forces using atomic force microscopy (AFM) ("Recording force events of single quantum-dot endocytosis").
|
|
Typically, investigations on QD–cell interactions involve injecting the nanoparticles into single cells using a glass micropipette. Other researchers have utilized small lipid bilayer-based pockets, or liposomes, that can merge with cell membranes to release the QDs into the cytoplasm. Unfortunately, these approaches lack spatial resolution and are unable to provide information on the dynamics of endocytosis, the process through which the living cells engulf the nanoparticles.
|
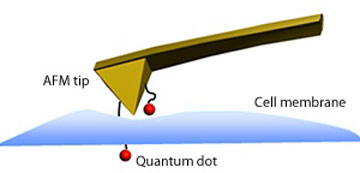 |
| Schematic representation of a quantum dot immobilized on an AFM tip penetrating the membrane of a cell.
|
|
Wang's team used AFM to evaluate these interactions in real time. They followed a fishing-inspired strategy in which QDs act as 'bait' at the end of an AFM 'fishing rod'. The researchers decorated the AFM probe with nitrogen-containing amino groups that they allowed to react with one extremity of a polyethylene glycol polymer chain. They then attached the QDs to the other end of this immobilized 'fishing line'.
|
|
The researchers assessed the performance of this QD-modified AFM tip by moving the tip toward model cells then away from them. The cells absorbed the nanoparticles upon contacting the approaching tip (see image), generating a force signal. Withdrawal of the tip produced a decreasing transient force, consistent with the dissociation of the QD from the cell.
|
|
Wang says that, unlike other researchers, his team focused on the trace peak of the AFM force curve. They discovered that this peak, which is acquired during the tip approach, provides important information on dynamic activity, in particular cellular uptake, and that its associated force was weaker than the unbinding force for a single QD. Wang believes that their strategy will bring new perspectives on the mechanism of interaction between 'nanoentities' and cells, and on the potential cytotoxicity of nanoparticles at single-particle levels.
|

