| Jun 29, 2011 |
Completely miscible nanocomposites - A breakthrough on its way to new types of functional materials
|
|
(Nanowerk News) In science and industry polymer nanocomposites are increasingly regarded as materials that will significantly help to define progress in the 21st century. They consist of a polymer matrix and of nanoparticles which are inserted into the matrix as filler materials. A research group led by Professor Stephan Förster of the University of Bayreuth has now developed a process which opens an avenue for the production of new, completely miscible nanocomposites. These materials represent an extremely varied potential for technological innovations. The scientists discuss their trail blazing development in the publication Angewandte Chemie International Edition ("Completely Miscible Nanocomposites").
|
|
Nanoparticles are minute particles having a diameter of less than 100 nanometers. They can be incorporated into polymer systems as filler materials. Unfortunately they have the tendency to aggregate within the polymer matrix. As such, they are not distributed as individual particles in all segments of the matrix, but rather form deposits in a limited number of locations in the matrix. The underlying cause for this behavior is that the nanoparticles need to exert significantly less interfacial energy in the aggregated condition, than if they existed in the polymer system individually.
|
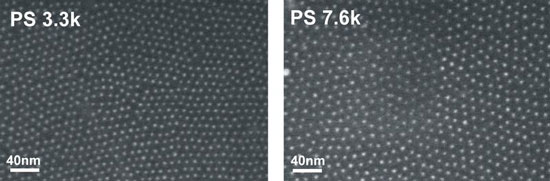 |
| Iron containing nanoparticles within a polymer matrix, as photographed with a raster electron microscope (SEM). The nanoparticles are prevented from aggregating with the aid of a polymer surface coating. The length of the polymer chains used for the surface coating determines the distance between the individual nanoparticles within the polymer system. Therefore, the distances can be regulated with a high degree of accuracy. PS 3.3k refers to polystyrol (a polymer system) with a molecular weight of 3300 g/mol, PS 7.6k refers to polystyrol with a molecular weight of 7600 g/mol.
|
|
However, for industrial applications, nanocomposites are much more attractive if the individual nanoparticles are distributed separately in the polymer system. In this case, the new materials are characterized by significantly better transparency, whereas aggregated nanoparticles cause them to be dull and opaque. Additionally, the electrical and thermal conductivity of the materials are more pronounced, the more uniformly the nanoparticles are distributed in the polymer system. Finally, the resulting materials are then also more heat and fire resistant.
|
|
But how can the aggregation of the nanoparticles in the polymer system be prevented? In an effort to solve this problem, Professor Stephan Förster, in cooperation with scientists of the University of Hamburg, has developed a new research idea which he has already implemented successfully at laboratory scale. The process begins with polymer chains. An adhesion molecule is attached to each chain. Just as with a grappling hook, the polymer chain attaches itself to a nanoparticle with the aid of this molecule; it does so in such a way that one end rests on the surface nearly vertically, whereas its other end points outwards. Using this method, each nanoparticle obtains a complete surface coating consisting of polymer chains, giving the coating the appearance of a spherical brush. These polymer chains, pointing outwards just as bristles do, prevent the nanoparticles from coming too close to each other as they are introduced into the polymer matrix. They are preserved as individual particles whereas the polymer chains are processed into the polymer system.
|
|
This opens the door for producing highly advanced functional materials, in which separate nanoparticles are incorporated into all sections of the polymer system. The characteristics and behaviors of these types of nanocomposites are largely dependent on the distance between neighboring nanoparticles. These distances can be regulated with great accuracy during production. The chemical composition of the nanoparticles can also vary, which has a profound impact on the resulting material. Consequently, this new process enables the targeted development of polymer nanocomposites which, based on their interior composition, exhibit specific characteristics and behaviors.
|
|
Semiconductor nanoparticles, e.g. those containing cadmium compounds, are of particular interest. If it were possible to comprehensively distribute these on an industrial scale into a polymer matrix, new perspectives would open up for the energy technology field. It so happens that nanocomposites of this type are likely to be suitable for the design of high performance solar cells, which are capable of converting a large portion of the stored light energy into electrical power. Also, apparently attractive are research activities on iron containing nanoparticles, which are incorporated into the polymer matrix at high densities. This would potentially result in very large capacities for magnetic storage of information in very dense spaces.
|
|
"In the coming years we intend to produce a broad spectrum of nanocomposites at laboratory scale and evaluate these for their application potential", declares Professor Stephan Förster. "I find it highly likely that this surface coating process will allow us to develop innovative functional materials which will surprise us with their exceptional performance characteristics."
|

