| Jul 11, 2011 |
Carbon nanofibers on graphene make promising high-performance electrode materials
|
|
(Nanowerk News) Lithium-ion batteries are an important energy storage technology for portable electronics and electric vehicles. The performance of these batteries depends on the density at which they can store energy and how fast they can be charged and discharged. One way to improve these properties is through the use of nanostructured electrodes. Zhuang-Jun Fan and colleagues from Harbin Engineering University and other institutions in China have now developed three-dimensional, carbon-based nanostructures with enhanced capacity for storing lithium ions and high-rate charge/discharge cycling characteristics ("Nanographene-Constructed Carbon Nanofibers Grown on Graphene Sheets by Chemical Vapor Deposition: High-Performance Anode Materials for Lithium Ion Batteries").
|
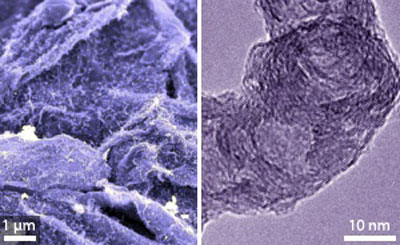 |
| A scanning electron microscopy image of a carbon nanofiber/graphene composite grown by chemical vapor deposition. (© ACS)
|
|
Graphene is a promising energy-storage material because of its high specific surface area and chemical stability. However, as Zhuang-Jun explains, graphene has not yet been used to its full potential in battery technologies because of the loss of surface area available for energy storage due to graphene aggregation. To address this issue, the researchers used a chemical vapor deposition approach in a fluidized bed reactor to grow one-dimensional carbon nanofibers on graphene nanosheets.
|
|
"The carbon nanofibers improve not only graphene aggregation," says Zhuang-Jun, "but also the overall conductivity of the graphene networks." The resulting graphene–carbon nanofiber hybrid structure contains many cavities, open tips and graphene platelets with exposed edges (see image), providing high capacity for lithium ion storage.
|
|
Zhuang-Jun and his co-workers grew the carbon nanofibers from nanoparticles of cobalt dispersed on sheets of graphene oxide. The cobalt nanoparticles act as catalysts for the growth of the nanofibers as the graphene oxide breaks up in the high-temperature growth reactor chamber. The outer shell of the resultant fibers consists of graphene platelets with a spacing of 0.342 nm. According to Zhuang-Jun, the graphene platelets arrange themselves roughly perpendicular to the fiber axis, which is favorable for the efficient diffusion of lithium ions. When used as an electrode in lithium-ion batteries, this structure allows for efficient charging and discharging processes, which occur through the insertion and extraction of lithium ions.
|
|
"The as-prepared hybrid material shows high reversible capacity, high-rate performance and cycling stability superior to pure graphene, natural graphite and carbon nanotubes," Zhuang-Jun remarks. These properties make the hybrid material a promising candidate as an electrode material for high-performance lithium-ion batteries, full cell and supercapacitors.
|

