| Jul 29, 2011 |
Battery in a nanowire is as small as energy storage can possibly get
|
|
(Nanowerk News) The world at large runs on lithium ion batteries. New research at Rice University shows that tiny worlds may soon do the same.
|
|
The Rice lab of Professor Pulickel Ajayan has packed an entire lithium ion energy storage device into a single nanowire, as reported this month in the American Chemical Society journal Nano Letters ("Building Energy Storage Device on a Single Nanowire"). The researchers believe their creation is as small as such devices can possibly get, and could be valuable as a rechargeable power source for new generations of nanoelectronics.
|
|
In their paper, researchers described testing two versions of their battery/supercapacitor hybrid. The first is a sandwich with nickel/tin anode, polyethylene oxide (PEO) electrolyte and polyaniline cathode layers; it was built as proof that lithium ions would move efficiently through the anode to the electrolyte and then to the supercapacitor-like cathode, which stores the ions in bulk and gives the device the ability to charge and discharge quickly.
|
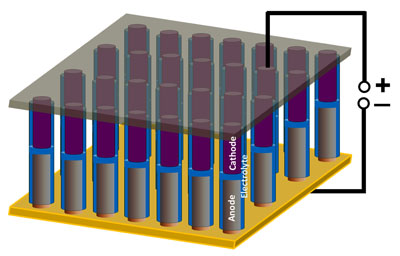 |
| A schematic shows nanoscale battery/supercapacitor devices in an array, as constructed at Rice University. The devices show promise for powering nanoscale electronics and as a research tool for understanding electrochemical phenomenon at the nanoscale. (Credit: Ajayan Lab/Rice University)
|
|
The second packs the same capabilities into a single nanowire. The researchers built centimeter-scale arrays containing thousands of nanowire devices, each about 150 nanometers wide. A nanometer is a billionth of a meter, thousands of times smaller than a human hair.
|
|
Ajayan's team has been inching toward single-nanowire devices for years. The researchers first reported the creation of three-dimensional nanobatteries last December. In that project, they encased vertical arrays of nickel-tin nanowires in PMMA, a widely used polymer best known as Plexiglas, which served as an electrolyte and insulator. They grew the nanowires via electrodeposition in an anodized alumina template atop a copper substrate. They widened the template's pores with a simple chemical etching technique that created a gap between the wires and the alumina, and then drop-coated PMMA to encase the wires in a smooth, consistent sheath. A chemical wash removed the template and left a forest of electrolyte-encased nanowires.
|
|
In that battery, the encased nickel-tin was the anode, but the cathode had to be attached on the outside.
|
|
The new process tucks the cathode inside the nanowires, said Ajayan, a professor of mechanical engineering and materials science. In this feat of nanoengineering, the researchers used PEO as the gel-like electrolyte that stores lithium ions and also serves as an electrical insulator between nanowires in an array.
|
|
After much trial and error, they settled on an easily synthesized polymer known as polyaniline (PANI) as their cathode. Drop-coating the widened alumina pores with PEO coats the insides, encases the anodes and leaves tubes at the top into which PANI cathodes could also be drop-coated. An aluminum current collector placed on top of the array completes the circuit.
|
 |
| An ultrathin battery/supercapacitor hybrid contains thousands of nanowires, each of which is a fully functional battery. The Rice University lab of Pulickel Ajayan developed the device. (Credit: Jeff Fitlow/Rice University)
|
|
"The idea here is to fabricate nanowire energy storage devices with ultrathin separation between the electrodes," said Arava Leela Mohana Reddy, a research scientist at Rice and co-author of the paper. "This affects the electrochemical behavior of the device. Our devices could be a very useful tool to probe nanoscale phenomenon."
|
|
The team's experimental batteries are about 50 microns tall -- about the diameter of a human hair and almost invisible when viewed edge-on, Reddy said. Theoretically, the nanowire energy storage devices can be as long and wide as the templates allow, which makes them scalable.
|
|
The nanowire devices show good capacity; the researchers are fine-tuning the materials to increase their ability to repeatedly charge and discharge, which now drops off after a about 20 cycles.
|
|
"There's a lot to be done to optimize the devices in terms of performance," said the paper's lead author, Sanketh Gowda, a chemical engineering graduate student at Rice. "Optimization of the polymer separator and its thickness and an exploration of different electrode systems could lead to improvements."
|


