| Aug 08, 2011 |
Metallic glasses: Some kind of order
|
|
(Nanowerk News) Metallic glasses are amorphous metals that are formed from the liquid state through rapid cooling, which prevents atoms from settling into an ordered crystalline structure. Bulk metallic glasses (BMGs) are a distinct class of metallic glasses that form out of multi-component alloys and are characterized by mechanical properties that are superior to conventional metals and alloys as well as a very high resistance to crystallization. However, the origin of this inherent reluctance of BMGs to crystallize has not been fully elucidated.
|
|
Qiang Wang, Yong Yang and co-workers from Shanghai University, the Hong Kong Polytechnic University and the City University of Hong Kong in China have now shown explicitly how a BMG develops local atomic clusters that prevent it from crystallizing on the macroscale ("Atomic-Scale Structural Evolution and Stability of Supercooled Liquid of a Zr-Based Bulk Metallic Glass").
|
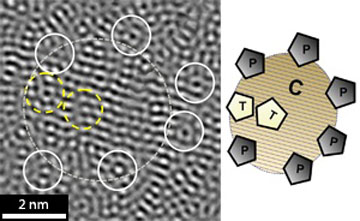 |
| High-resolution transmission electron microscopy image (left) showing the formation of icosahedra-like atomic clusters, and a schematic illustration of cluster formation (right). T, trapped clusters (yellow outline); P, pinning clusters (solid white outline); C, crystalline phase (broken white outline).
|
|
The researchers studied a zirconium-based alloy that is known to have high thermal stability, allowing it to resist crystallization even when heated, as well as a very low cooling rate that provides a broad time window for studying the mechanism of glass formation. "One of the questions that puzzled us was how some BMGs can attain such high thermal stability," says Yang.
|
|
Using a combination of X-ray diffraction and high-resolution transmission electron microscopy, the researchers studied the evolution of the atomic structure of the Zr41.2Ti13.8Cu12.5Ni10Be22.5 alloy during isothermal annealing. The as-prepared samples were amorphous, but within an hour complex icosahedral atomic clusters started to form (see image), and subsequent annealing resulted in the development of stable crystalline phases. However, the material did not develop the long-range crystalline order needed to form a macroscale crystalline structure.
|
|
"To achieve long-range translational symmetry, the crystalline phases need to grow out of their embryonic state." explains Yang. However, the presence of the atomic clusters prevents that from happening. "On the one hand, these icosahedra-like atomic clusters behave like pinning particles around the periphery of a crystalline phase. On the other, they cause lattice distortions within the crystalline phase, raising the energy barrier against further crystal growth," he says. According to Yang, the observed behavior may be related to how atoms of different sizes in a multicomponent system rearrange in a manner that optimizes packing efficiency.
|

