| Sep 29, 2011 |
Full to the brim with hydrogen
|
|
(Nanowerk News) Hydrogen could be one of the most important fuels in a new energy economy based on renewable resources. However, no ideal hydrogen storage material has yet been found. A team led by Yaroslav Filinchuk at the Université Catholique de Louvain, Belgium, and Torben R. Jensen at the University of Aarhus in Denmark has now introduced a new highly porous form of magnesium borohydride in the journal Angewandte Chemie ("Porous and Dense Magnesium Borohydride Frameworks: Synthesis, Stability, and Reversible Absorption of Guest Species"). This material can store hydrogen in two ways: chemically bound and physically adsorbed.
|
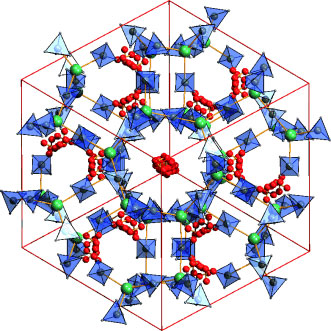 |
| Highly occupied: A highly porous form of Mg(BH4)2 (see picture; Mg green, BH4 blue, unit cells shown in red) reversibly absorbs H2, N2, and CH2Cl2. At high pressures, this material transforms into an interpenetrated framework that has 7% higher density than the other polymorphs. Mg(BH4)2 can act as a coordination polymer that has many similarities to metal-organic frameworks. (© Wiley-VCH)
|
|
The perfect hydrogen storage material must store hydrogen efficiently and securely in a small volume, and should release it on demand. It must be rapidly refillable under mild conditions, while being as light and inexpensive as possible. One approach to this is solid-state storage. In such systems, hydrogen can be chemically bound, as in borohydride compounds, or it can be adsorbed as a molecule into a nanoporous material, as in some metal–organic frameworks.
|
|
The researchers have now found a material that can do both. It is a new, highly porous form of magnesium borohydride—the first light-metal hydride that is porous like a metal–organic framework and is capable of storing molecular hydrogen.
|
|
Magnesium borohydride (Mg(BH4)2) is one of the most promising materials for chemical hydrogen storage because it releases hydrogen at relatively low temperatures and can hold a high proportion by weight (about 15%) of hydrogen. Two forms of this compound, α and β, were previously known. The researchers have now made a third form, designated the γ form. Its pore volume comprises about 33% of the structure, and its channels are wide enough to take up and store small gas molecules, such as nitrogen, dichloromethane, and most importantly hydrogen.
|
|
Interestingly, under high pressure this material converts into a nested, non-porous framework with a density that is nearly 80% higher. This makes the δ form the second densest in hydrogen content and more than twice as dense as liquid hydrogen. Furthermore, this conversion results in a 44% reduction in volume, which is the largest contraction yet observed for a hydride.
|
|
"A combination of the chemical (through covalent bonding) and physical (through adsorption in the pores) storage of hydrogen seems to be difficult in practical applications," explains Filinchuk. "However, this research has a broader impact, as it reveals a new class of hydride-based porous solids for storage and separation of various gases."
|

