| Posted: December 8, 2007 |
Chemists make fullerene necklace |
|
(Nanowerk News) Spanish scientists have strung fullerene buckyballs together to produce a polymer with unique electronic properties. The creation of these polymers, which resemble a string of pearls, has demonstrated a new approach to designing novel materials.
|
|
The spherical fullerene C60 contains 60 carbon atoms in the arrangement of a soccer ball. Although these molecules have remarkable electronic properties, it has been difficult to put them to practical use.
|
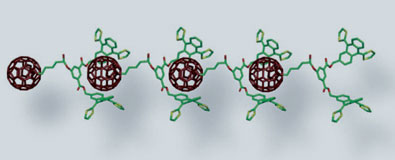 |
| A specially designed receptor for C60 fullerenes. The monomer self-assembles in a head-to-tail fashion
|
| \
Now, a team led by Nazario Martin at Ciudad Universitaria in Madrid, Spain, have devised a new way to trap buckyballs in polymers ("Self-Organization of Electroactive Materials: A Head-to-Tail Donor-Acceptor Supramolecular Polymer"). The key lies in the design of the monomer - a complicated series of joined rings that act like a 'pincer', gripping the buckyball tightly through non-bonding interactions.
|
|
'Our new supramolecular polymers show organization in solution as well as in the solid state,' said Martin. 'We believe this could be useful for applications such as organic photovoltaic solar cells.'
|
|
The monomer was created by extensively modifying the structure of tetrathiafulvalene, a heterocyclic compound containing four sulphur atoms. The resulting conjugated aromatic structure is concave and holds the neighbouring buckyball securely, allowing the fullerenes to link up in a chain.
|
|
The team designed their monomer to self-assemble in a head-to-tail fashion and were able to grow lengths of over 100 repeating units, up to 300nm in length. They analysed their polymers using atomic force microscopy and reported 'long, winding, necklace-like fragments'. At approximately 2nm wide, these polymers are about 5 million times smaller than a real set of pearls.
|
|
Importantly, the buckyball-containing polymers are redox-amphoteric - they can both accept and donate electrons. That could make the polymers useful for electronic devices - a possibility the team plans to investigate further.
|
|
Stuart Rowan, an expert on supramolecular polymers at Case Western Reserve University, Cleveland, US, told Chemistry World: 'This is a neat system and the fact that this supramolecular polymer is electroactive is potentially quite useful. It will be interesting to see how this develops.'
|

