| Posted: December 21, 2007 |
Capped carbon nanotubes as chemical couriers |
|
(Nanowerk News) US scientists have reported a mild new method for trapping liquids and nanoparticles inside carbon nanotubes.
|
|
Alexander Yarin's team at the University of Illinois at Chicago, US, have developed a room-temperature method to fill carbon nanotubes with liquids ("Room-temperature, open-air, wet intercalation of liquids, surfactants, polymers and nanoparticles within nanotubes and microchannels" – open access article).
|
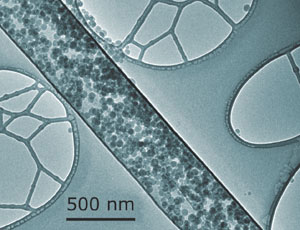 |
| Capped nanotubes could be used for drug delivery
|
|
The filling of carbon nanotubes with aqueous solutions can have biomedical uses, as Yarin explained, 'Nanotubes with diameters of the order of 100 nm are possible drug carriers, which can deliver biological payloads to a certain location, such as a tumour.'
|
|
In Yarin's technique, water is dragged into nanotubes by a self-sustained diffusion mechanism. A toluene solution of a polymer, in this case polycaprolactone, is then pulled into the nanotubes. As the polymer is insoluble in the water already in the tubes, the polymer gathers at the ends and forms caps. As a result, the water becomes trapped within the nanotubes. Crucially, this takes place under mild conditions, which is where this method holds its advantage according to Yarin: 'existing filling methods involve high pressures or temperatures, which are detrimental to biologically-active materials'.
|
|
Marc in het Panhuis, a senior lecturer at the University of Wollongong, Australia, forecasted how this technique may avoid current problems involved with using nanotubes for drug delivery: 'This is an elegant way of tuning the properties of nanotubes from within, while the outer surface can be modified to render the nanotube biocompatible'.
|
|
Surfactants and particles, such as polystyrene nanospheres, have also been trapped inside carbon nanotubes using this method. This means that the technique could have multiple other future uses, Yarin suggested, such as in 'catalysis, supercoolants, optoelectronics and sensors'.
|

