| Posted: March 28, 2008 |
Two-photon nanoparticles for the improved detection of tumor cells |
|
(Nanowerk News) Researchers from several CNRS-associated laboratories(1) have succeeded in synthesizing porous nanoparticles that are capable of absorbing the energy of two photons in the near infrared spectrum, and then re-emitting radiation used for medical imaging by fluorescence. These two-photon nanoparticles should enable the more precise detection of tumor cells and, in the longer term, better-targeted therapy. These results, already made available on-line by Chemistry of Materials, were published on March 25, 2008 ("Synthesis and Characterization of Fluorescently Doped Mesoporous Nanoparticles for Two-Photon Excitation").
|
|
At present, the medical imaging of tumor cells is based on the fluorescence emitted by chemical groups that can absorb the energy of a photon. These molecules, called fluorophores, are excited in the visible ultraviolet spectrum. Single-photon imaging thus remains relatively imprecise. This obstacle should soon be overcome thanks to work by scientists from CNRS-associated laboratories (1).
|
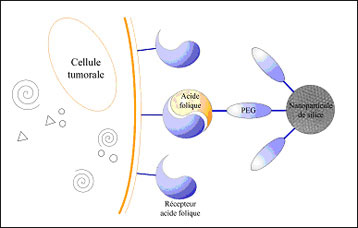 |
| Diagram explaining the recognition of tumor cells by nanoparticules (Image: ICGM).
|
|
These researchers have succeeded in developing organic, two-photon fluorophores (aromatic molecules) that are able simultaneously to absorb two photons in the near infrared spectrum. These were then encapsulated in porous nanoparticles to enable their circulation in a biological medium. The originality of this work resides in the fact that unlike ultraviolet wavelengths, infrared wavelengths penetrate more deeply into tissues and are less energetic, the advantage being that they can explore tumors more profoundly without damaging the tissues. Furthermore, the use of two-photon fluorophores favors access to a 3D spatial resolution, which in the longer term will enable the detection and more targeted treatment of tumor cells. One of the options envisaged may be to encapsulate in the pores of silicon nanoparticles not only the fluorescent agent but also drugs that can locally treat the cancer cells.
|
|
The scientists have also been focusing on the functionalization of these nanoparticles in order to create new biological markers capable of interacting with breast and cervical cancer cells. To achieve this, they grafted on the nanoparticles a monolayer made up of a hydrophilic polymer (PEG: polyethylene glycol) and folic acid. The latter forms the ligand (2) recognized by the receptors of HeLa cells (cervical cancer) and MCF7 cells (breast cancer) (see diagram). These results should enable the 3D targeting and imaging of the tumor. Other functionalizations could be envisaged, enabling the detection of other tumors.
|
|
Notes:
|
|
1) Institut Charles Gerhardt Montpellier (CNRS / Université de Montpellier 2); Institut européen des membranes (CNRS / Université de Montpellier 2); Laboratoire de chimie et photonique moléculaires (CNRS / Université de Rennes 1); Institut de chimie de la matière condensée de Bordeaux (CNRS) (*) and Laboratoire physico-chimie, pharmacotechnie et biopharmacie (CNRS / Université de Paris 11 Paris-Sud) (**).
(*) David N'Guyen, Etienne Duguet (**) Catherine Dubernet, Delphine Méthy-Gonnod.
|
|
2) Molecule binding to the active site of a protein. This binding generally triggers a biological response.
|

