| Posted: December 18, 2008 |
Next generation microscopy: No stain, big gain |
|
(Nanowerk News) Microscopes have revolutionized the practice of science, especially in the fields of biology and medicine. Just a few hundred years ago, gaining the ability to study what was previously unobservable opened up an entirely new world. Today, imaging techniques remain indispensable to clinicians and researchers who regularly diagnose medical conditions and work to develop new treatments.
|
|
Test results can often take hours or even days because cells or tissues must be subjected to lengthy fixation and labeling processes, sometimes called staining, in order to visualize and distinguish cellular components. In addition to long processing times, staining procedures often include harsh treatments or conditions that alter the tissues themselves, making interpretation of results difficult.
|
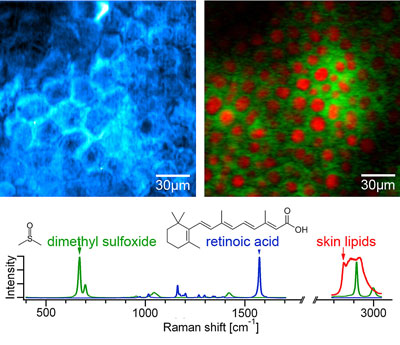 |
| This figure shows SRS images for diffusion of retinoic acid (blue) and DMSO (green) through the top layer of the skin, or stratum corneum, which is the main barrier against topically applied drugs. Retinoic acid, a common drug for acne, diffuses through the lipids of the stratum corneum (polygonal shape), and is visualized by tuning the Raman shift into the characteristic band at 1570cm-1 (blue). DMSO, often used as a diffusion enhancer, goes deeper into the skin. It is hydrophilic (water-loving) so it avoids the lipid structures that retinoic acid diffuses through readily. This is highlighted using two-color SRS imaging tuned into the characteristic vibration of DMSO at 670cm-1 (green) and the vibration of typical skin lipids at 2845cm-1 (red) at a depth of ∼65µm into the skin. (Image: Image Courtesy of Chris Freudiger, Wei Min, Brain Saar, Harvard University, in collaboration with Pfizer's Jason Tsai)
|
|
A newly developed label-free imaging technique called stimulated Raman scattering (SRS) will likely revolutionize biomedical imaging in research and diagnostic laboratories. A team lead by Sunney Xie at Harvard University reported this new technique in the December 19 issue of Science.
|
|
"It is a big step forward in terms of biology," said Xie. "SRS is a powerful imaging modality with widespread applications on many fronts of biology and medicine. This work compliments an earlier technique we developed with funding from the National Science Foundation, adding a new imaging modality to the vibrational microscopy field."
|
|
The key to this new chemical imaging technique is the use of two lasers with different frequencies. Researchers visualize samples by tuning the laser frequencies to match the vibrational frequency of a specific chemical bond. Each type of molecule within a sample, including nutrients or drugs, is detectable at a unique frequency. By combining sample data collected at numerous frequencies, researchers can produce a high-resolution 3D image of the sample. SRS microscopy represents a big gain in biomedical imaging because it avoids labor-intensive sample preparation and autofluorescence, or "background noise", associated with traditional fluorescence microscopy.
|
|
Xie is enthusiastic about the ways in which SRS imaging will facilitate progress in many fields. "Applications of SRS imaging range from mapping distribution of small metabolite and drug molecules in cells and tissues to medical diagnosis of cancer. Neuroimaging is another exciting area of application."
|

