| Posted: January 25, 2009 |
Hydrogen bonding instrumental in self-organizing complex nanostructures |
|
(Nanowerk News) Nicola Armaroli and co-workers from CNR-ISOF, Bologna, Italy, and Davide Bonifazi and colleagues from the Università di Trieste, Italy, and the University of Namur, Belgium, have shown that pi-conjugated molecules bearing complementary hydrogen bonding sites can self-organise into complex nanostructures, which resemble natural micellar systems ("Engineering spherical nanostructures through hydrogen bonds" – free access article).
|
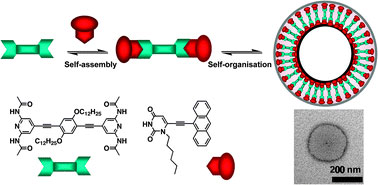 |
| Chromophoric acetylenic scaffolds bearing complementary uracyl and 2,6-di(acetylamino)pyridyl moieties undergo supramolecular recognition and generate uniform nanoparticles
|
|
Nature creates spectacular nanoarchitectures through specific supramolecular assemblies of various components. Complementary hydrogen bonds are often utilised, which leads to the optimization of solvophilic interactions.
|
|
The use of hydrogen bonding also enables the tuning of the size and shape of the nanoparticles. The complementary hydrogen bonds promote self-organisation of the nanoparticles into uniform aggregates, and also enable a morphological change to occur from nanoparticle to vesicles.
|
|
Armaroli has also shown that the nanoaggregation can be reversed with temperature, which suggests possible applications of these vesicles in molecular delivery.
|
|
'The ultimate aim of this work is to create a library of nanoarchitectures, which may exhibit potential applications as drug carriers, in biological imaging and in optoelectronic devices,' says Armaroli.
|
|
However, Armaroli acknowledges that one of the main challenges to overcome is to design nanostructures with the desired molecular functionality, without compromising key features such as chemical stability and photoluminescence.
|

