| Posted: February 26, 2009 |
MOFs that can separate different gases |
|
(Nanowerk News) Scientists have developed a new type of porous material that could be used to separate mixtures of different gases.
|
|
The material’s structure is based on a three-dimensional lattice of aluminum atoms, surrounded by oxygen atoms. These clusters are connected by a carbon-based molecule called 1, 4-naphthalene dicarboxylate (1, 4-NDC). Such metal–organic frameworks (MOFs) are finding uses in separating liquids or gases, acting as catalysts to speed up chemical reactions, or for safely storing large volumes of gas.
|
|
The international team of scientists that made the material, including Masaki Takata of RIKEN’s Harima Institute, used x-rays to reveal that it contains ordered channels of two different sizes (Nanochannels of two distinct cross-sections in a porous Al-based coordination polymer). The smaller channels, crowded by napthalene units, are just 0.3 nanometers (billionths of a meter) across, while the larger channels are 0.77 nanometers across.
|
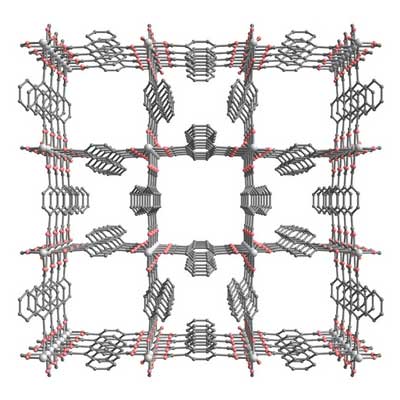 |
| A schematic of the metal-organic framework, which contains a highly ordered network of small and large nanopores.
|
|
“We expected the porous structure to some extent,” says Satoshi Horike, now at the University of California, Berkeley, who was part of the team. “But the existence of two kinds of pores in our system is kind of surprising.”
|
|
The team then used nuclear magnetic resonance (NMR), a technique which measures the magnetic behavior of atomic nuclei, to build up a more detailed three-dimensional picture of the MOF.
|
|
The scientists analyzed a sample of the inert gas xenon as it diffused through the MOF’s pores. This experiment confirmed that the material has parallel, yet independent, pores. Xenon atoms have a diameter of about 0.44 nanometers, and did not fit down the smaller channels. However, it took mere milliseconds for the xenon to diffuse through the larger channels, which were not blocked by any other molecules, such as water.
|
|
Carbon dioxide could also seep through the material. The scientists calculate that each gram of the MOF can bind carbon dioxide to an internal surface area which is greater than 500 m2, roughly the size of two tennis courts.
|
|
And while the small organic molecules methanol and acetone were easily absorbed, more pressure was required to squeeze water into the pores because of high hydrophobicity. This could make the material useful for separating water from alcohols, suggests Horike. “Gas separation between water and ethanol is important for obtaining liquid fuel from biomass sources,” he says.
|
|
The smaller pores could also be useful for storing hydrogen gas, a potentially environmentally friendly fuel. “Hydrogen has a really small interaction with pore surfaces,” explains Horike, “and the small pore would be able to bind hydrogen gas more tightly than the large one.”
|

