| Dec 13, 2012 |
International collaboration reveals how cell membranes reassemble after cell division
|
|
(Nanowerk News) An international collaboration between researchers at the Babraham Institute, Cancer Research UK's London Research Institute, Imperial College London and Amherst College in the US, has revealed an instrumental molecule in ensuring that the nuclear membrane reforms correctly after cell division, and therefore plays a key role maintaining the delicate balance between cell growth and cell death.
|
|
The molecule, called diacylglyercol (DAG) is a type of lipid, a group of naturally occurring molecules that include fats, waxes, sterols, and fat-soluble vitamins. While gaining new insight into the basic mechanisms underpinning cell growth and communication within cells, this research may also point towards unexplored therapeutic avenues to combat the abnormal cell division seen in certain types of cancer and rare genetic disorders.
|
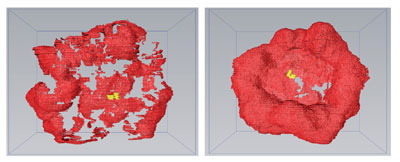 |
| Comparison of 3D models of the nuclear envelope reconstructed from correlative light and electron microscopy (CLEM) serial images, of diacylglycerol-depleted (DAG) (left panel) and DAG-rescued (right panel) HeLa cells shows the NE reformed in the presence of DAG. (Image: Babraham Institute)
|
|
During cell division the membrane around the cell nucleus (nuclear envelope) breaks down and reforms around the two daughter cells after each division. For cells to stay alive and regulate their communication from the nuclear compartment to other parts of the cell, the nuclear envelope must reform correctly around the genetic material. If it does not form properly, human diseases such as cancer can arise. The precise mechanisms governing this membrane transformation have remained elusive. The majority of research investigating how the nuclear envelope is assembled has suggested that proteins are the sole molecules responsible for safeguarding its formation. However, this recent research, reported online in the open access Journal PLoS ONE ("Acute manipulation of diacylglycerol reveals roles in nuclear envelope assembly & endoplasmic reticulum morphology"), pinpoints a particular type of lipid (DAG) as playing the key role in nuclear membrane reformation that this does not depend on proteins.
|
|
Professor Michael Wakelam, co-author of the paper and Director of the Babraham Institute, which receives strategic funding from the Biotechnology and Biological Sciences Research Council (BBSRC) explained, "Without affecting other cellular compartments, we have locally removed this lipid, resulting in a fragmented nuclear envelope and cell death. The cells, which did not have DAG removed, however, lived and continued dividing. By adding back this one type of lipid to DAG-depleted cells, the nuclear envelope reformed in its entirety and the cell survived through further divisions."
|
|
This study provides for the first time in vivo evidence for a structural role of DAG in shaping a cell's organelles. It also demonstrates that proteins are not the sole regulators in nuclear envelope formation. DAG appears to play an important role in places where there is extreme membrane curvature required for fusion, for which proteins alone are an insufficient explanation. While proteins play a key role in membrane structure and function, events like membrane fusion or organelle shaping require asymmetric alterations of lipid composition across the membrane.
|
|
Professor Banafshé Larijani, senior author of the paper says, "Our findings also reveal that these molecular events are highly conserved, from sea urchin embryos to mammalian cells. These studies have reset the sea urchin at centre stage as a model organism for various studies in Cell Biology and Signal Transduction."
|
|
The Babraham Institute is a world-leading centre for studying the basic biology of signalling processes, including those involving lipids, inside and between cells, supporting BBSRC's mission to drive advances in fundamental bioscience for better health and improved quality of life.
|
|
Professor Wakelam added, "Our investigations have forged a new path in locally targeting lipid synthesis pathways, specifically DAG synthesis. During abnormal and excessive cell growth the localised prevention of DAG synthesis may result in restraining unregulated cell growth, which if not obstructed could develop into various types of cancers. The Institute's lipidomics facility, which has been supported by strategic funding from BBSRC, is enabling us to analyse the importance of lipid signalling molecules in a range of developmental processes and to explore the basis of diet-related ill health. A better understanding of the way the nuclear membrane forms when cells divide could provide new insight for treating these disorders."
|
|
A recent investment of £1.8M by BBSRC has enabled a significant enhancement of the Institute's Mass Spectrometry Facility with the purchase of five new mass spectrometers, which are used to analyse almost any type of biomolecule including proteins ('proteomics') and lipids ('lipidomics'). This service and 'enabling technology' is available for outside academic and commercial collaboration, resonating with BBSRC's commitment to develop strategic partnerships and make available facilities to HEIs and the life science industry.
|

