| May 29, 2013 |
Micelle microscopy
|
|
(Nanowerk News) The first high-resolution view of micellar bundles formed from a solution of wormlike micelles has been made possible by sample preparation techniques developed at EMSL and EMSL’s suite of electron microscopes ("Microstructure and rheology of a flow-induced structured phase in wormlike micellar solutions").
|
|
Micelles are formed from surfactant molecules, which are hydrophilic on one end and hydrophobic on the other. In water-based solutions, surfactant molecules can self-assemble into micellar structures such as sheets, spheres, and cylinders that are hydrophilic on the outside and hydrophobic at the core. This property makes micelles useful for applications such as oil recovery and drug delivery. For example, micelle cores can carry fat-soluble medications.
|
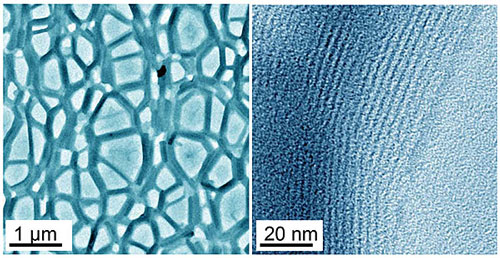 |
| EMSL provided the first-ever high-resolution view of micellar bundles formed from a wormlike micelle solution. CryoTEM images above show FISP branches of micellar bundles (dark) and pores (light) with their astoundingly aligned ultrastructure.
|
|
To better understand how to tailor the physical properties of micelles for target applications, a team of EMSL users studied the effects of surfactant concentration, salt concentration, and physical stress on two flexible cylinder—or wormlike—micellar solutions. Under controlled forcing through an array of microposts in a well-defined chemical environment, each wormlike micelle solution formed a network of multi-connected micelle bundles called a flow-induced structured phase (FISP). Each FISP had dramatically different mechanical properties than its precursor solution—an effect not unlike churning cream into butter.
|
|
Scanning electron microcopy, transmission electron microscopy, cryogenic transmission electron microscopy, and rheological analyses of each solution and its resulting FISP revealed that under the physical stress of the micropost array, the lower concentration, less viscous solution became a highly porous but relatively dense gel. In contrast, the higher concentration, more viscous solution became a highly porous and forgiving gel—an unintuitive result that will help guide micellar design.
|

