| Jul 16, 2015 |
Light-gated control of cell division
|
|
(Nanowerk News) Chemists from the Ludwig-Maximilians-Universität München (LMU) have developed photoresponsive derivatives of an antimitotic drug, which permit light-dependent control of cell division. The new agents could provide the basis for precisely targeted tumor therapies, free of side-effects.
|
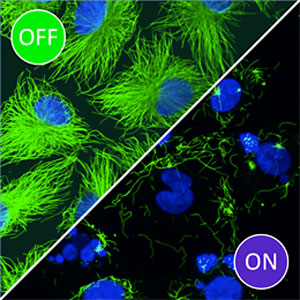 |
| In cells incubated with Photostatins in the dark (upper left, OFF), microtubules (green) and cell nuclei (blue) are clearly unaffected. However, exposure to blue light (lower right, ON) leads to disintegration of the microtubules, which results in breakdown of the nuclei and ultimately in cell death. Source: Dirk Trauner/LMU
|
|
The cells of higher organisms possess three dynamically modifiable systems of protein filaments, collectively referred to as the cytoskeleton, whose elements play crucial roles in fundamental cellular processes. One of these systems consists of fibrous polymers called microtubules, which are in turn made up of globular subunits called tubulins. As well as serving as highways for intracellular transport, microtubule assemblies form the spindle apparatus that is responsible for the ordered segregation of chromosomes to the daughter cells during cell division (mitosis).
|
|
Hence, compounds that bind specifically to microtubules provide vital tools for research both on cell reorganization and cargo trafficking, as well as the regulation of mitosis and the pattern of embryonic development. Such agents also find application as inhibitors of the abnormal proliferation that characterizes tumor cells, although they have serious drawbacks, in that they also kill healthy cells in specialized tissues that also need to divide quickly (bone marrow, gastrointestinal tract, etc).
|
|
However, research led by Professor Dirk Trauner and Dr. Oliver Thorn-Seshold at LMU‘s Department of Chemistry has developed an innovative chemical strategy which allows their inhibitory effect to be localized to a selected tissue site, and so should avoid the collateral damage that such drugs normally cause.
|
|
“We have incorporated a light-sensitive molecular switch into a known inhibitor of microtubule polymerization, so that the agent only becomes active when it is exposed to blue light. This makes it possible, for the first time, to restrict its action to particular sites – and to inactivate it at will, since the reaction is reversible,” Thorn-Seshold explains. “With this optical switch, we have extended the range of photopharmacology to yet another highly dynamic functional system that is common to all multicellular organisms – the cytoskeleton,” Trauner adds.
|
|
Compounds that bind to microtubules are among the most potent types of drug used in cancer chemotherapy. However, because they affect essentially all actively dividing cells, these agents cause severe side-effects.
|
|
“Our aim was to modify a microtubule inhibitor in such a way that its action could be limited to a targeted tumor tissue,” says Thorn-Seshold, “and we have now achieved this goal by designing a suitable light-sensitive molecular switch for several chemical derivatives of the bioactive agent colchicine.”
|
|
Colchicine is a toxic natural product that was first isolated from the meadow saffron Cochicum autumnale, and Trauner and his team refer to their synthetic photo-switchable derivatives of the compound as Photostatins. Photostatins are inactive in the dark, but can be converted into active inhibitors of microtubule polymerization by illuminating them with a pulse of blue light. This feature permits precise spatial control of their action, designed to avoid off-target effects on healthy cells in other tissues.
|
|
A STOP light for tumor cells
|
|
In laboratory tests, cells treated with Photostatin and illuminated with blue light were efficiently killed, while cells incubated with increasing amounts of the same drug in the dark suffered no ill effects until a ~250-fold higher dose was applied. Therefore, tissues incubated with an intermediate dose of Photostatin can be either destroyed or left unharmed, depending on whether they are exposed to blue light or not.
|
|
“This degree of light-induced activation is unprecedented in photopharmacological experiments,” says Trauner, “and that can be attributed to our use of a new design principle, which allows for much more powerful control over the compounds’ bioactivity.” The work, which was carried out in collaboration with colleagues at the University of Lyon, as well as teams led by Angelika Vollmar and Stefan Zahler and by Markus Rehberg at LMU, appears in the leading biological journal Cell ("Photoswitchable Inhibitors of Microtubule Dynamics Optically Control Mitosis and Cell Death").
|
|
The researchers believe that Photostatins would be most likely to find clinical application in the treatment of localized tumors that are easily accessible to light, such as retinoblastoma – the most common pediatric tumor of the eye – and skin cancers.
|
|
“However, fiber optics are already in routine use in medical procedures such as endoscopic examination of the gastrointestinal tract. And given the pace of development in LED-based technology, miniaturized and more powerful LEDs that can be implanted in the body – like tiny, blinking pacemakers – are probably not that far off,” says Thorn-Seshold. “We hope that our photosensitive drugs will help to stimulate the search for such innovative therapeutic approaches. This, however, will be a long-term process, as the design and implementation of the necessary experimental studies will certainly take years.”
|
|
Timing developmental trajectories
|
|
Be that as it may, the Photostatins are already providing cell biologists with a powerful new tool. For microtubules not only play a crucial part in cancer progression, they also play complex roles in the transport of signaling molecules, cargoes, and organelles around cells, as well as in cell movement, migration and repair, and are critical for the organization of embryonic development.
|
|
Since Photostatins enable exquisitely precise spatial and temporal control over microtubule dynamics – enabling control over individual cells within networks, and with switching times under a second – they open up new ways of exploring how the functions and effects of microtubules are regulated in space and time.
|
|
“One could, for instance, arrest the differentiation of a selected cell over a given time period, then remove the brake and observe its recovery and how it integrates into the further development of an organism. This promises to give us new insights into the role of various types of progenitor cells during development,” Trauner says.
|
|
Photopharmacology is still a young field, but it seems set to become ever more significant in the coming years. Trauner’s group now intends to add light-modulatable switches to other molecules involved in regulating the mechanics and dynamics of cell division: optical control of microtubule function is only the beginning.
|

