| Feb 20, 2017 |
Unlocking peptide potential
|
|
(Nanowerk News) Peptides are naturally occurring molecules with excellent pharmaceutical properties. However, their therapeutic use has been limited by the relatively small number of natural peptide structures.
|
|
Powerful new computational methods now enable scientists to design a virtually unlimited variety of hyperstable peptide structures not found in nature ("Accurate de novo design of hyperstable constrained peptides"). This research opens a new frontier in drug discovery.
|
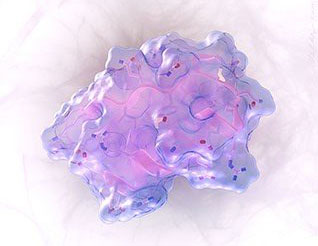 |
| The molecular surface of this peptide is shown as a transparent blue shell, and the peptide’s backbone structure is pink. The amino acid side chains are white (carbon atoms), blue (nitrogen atoms), and red (oxygen atoms). The crisscrossing bonds that give the peptide its constrained, stable shape are in bright white. (Image: Vikram Mulligan, University of Washington)
|
|
Designing custom peptides that fold into previously inaccessible regions provides the foundation for developing a new generation of peptide-based drugs. These drugs would have enhanced pharmacological properties and would be safer, cheaper, and more effective than conventional therapeutics.
|
|
Most drugs currently approved for humans are either large proteins or small molecules. Between these two size categories are peptides. Peptides are molecules with excellent pharmaceutical properties because they combine the stability and tissue penetration of small-molecule drugs with the specificity of much larger protein therapeutics.
|
|
However, naturally occurring peptide structures are limited in variety. This limitation underscores the need for novel, powerful computational approaches to design a wide range of peptides to fully explore their potential for drug discovery.
|
|
To address this need, a multi-institutional team of researchers developed new computational methods that predict how a string of amino acid residues folds into a three-dimensional structure, enabling the de novo design of peptides with a broad range of previously inaccessible sizes, shapes, and functions.
|
|
To show their computational design methods worked, the researchers synthesized a subset of de novo peptides that were 18 to 47 amino acid residues in length with diverse topologies.
|
|
The introduction of chemical features constrained their shape and made the new peptides hyperstable, resistant to extreme temperatures and exposure to harsh chemicals.
|
|
To show these peptides folded as designed, the team determined the structures for 12 of the synthesized peptides with the assistance of data collected on the high-field nuclear magnetic resonance (NMR) spectrometers at the U.S. Department of Energy’s (DOE) Environmental Molecular Sciences Laboratory (EMSL), a DOE Office of Science user facility.
|
|
The experimentally determined X-ray and NMR structures were nearly identical to those determined from the computational models. The ability to precisely control the size and shape of peptides designed to fit into a specific target-binding pocket has potential to unlock a new generation of peptide-based therapeutics to treat a wide variety of diseases.
|

