| Jul 19, 2017 |
Control of the unfolded protein response in health and disease
|
|
(Nanowerk News) Information generated by screening tools, readily available therapies and potential pathways to drug development are the cornerstone of informed clinical research and clinical trial design. In a new review in the August 2017 issue of SLAS DISCOVERY (formerly the Journal of Biomolecular Screening), authors Eric Chevet, Ph.D., of Inserm U1242 (Rennes, France) et al. analyze the recent literature and review the impact of unfolded protein response (UPR) in health and disease ("Control of the Unfolded Protein Response in Health and Disease").
|
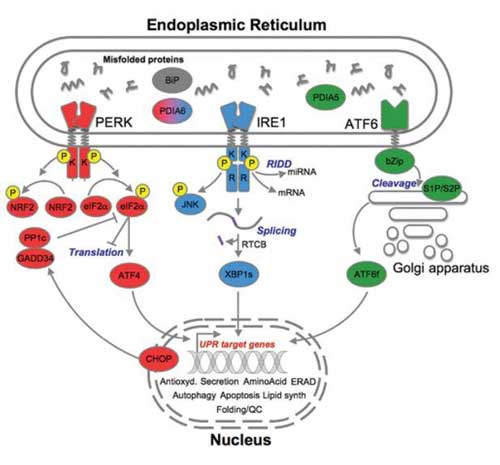 |
| The three endoplasmic reticulum (ER) stress sensors (PERK [red], IRE1 [blue], ATF6 [green]) initially activate signaling events that increase protein-folding capacity and reduce protein load on the ER. These transcriptional and translational outputs tend to reestablish protein-folding homeostasis in the ER and promote cell survival. (Image: Eric Chevet et al.)
|
|
In addition to providing an in-depth description of the molecules found to target the three arms of UPR, the review provides an overview of tools available for the screening and development of novel therapeutic agents that can modulate the UPR for future disease intervention.
|
|
The UPR is activated in response to endoplasmic reticulum (ER) stress. ER is the first compartment of the secretory pathway in eukaryotic cells. Its main functions comprise calcium and lipid homeostasis maintenance as well as the productive folding of secretory and transmembrane proteins. When the protein synthesis and folding demand exceeds the ER capacity, improperly folded proteins accumulate in this compartment, thus leading to a situation called ER stress.
|
|
To cope with this imbalance, the cell activates the UPR. The UPR is an integrated adaptive biochemical process that is inextricably linked with cell homeostasis and paramount to the maintenance of normal physiological function. Prolonged ER stress can push the UPR past beneficial functions, such as reduced protein production and increased protein folding and clearance to apoptotic signaling. When this occurs, the UPR contributes to the commencement, maintenance and exacerbation of a multitude of disease states, making it an attractive global target for tackling conditions sorely in need of novel therapeutic intervention.
|

