| Apr 12, 2023 |
Hammock for brain organoids
|
|
(Nanowerk News) Nerve cells communicate through chemical signals (neurotransmitters), which are converted into electrical signals that pass information from one nerve cell to the next. This is also the way in which the neurons in the brain organoids communicate with each other.
|
|
"To find the causes of various brain diseases and new therapeutic approaches, it is not enough to simply look at nerve cells under the microscope. You also need to know how the nerve cells work - how they communicate with each other," says Thomas Rauen. However, current systems for recording the communication between nerve cells in brain organoids have their limitations. In the relatively large brain organoids, the sensors either do not get close enough to the nerve cells or they destroy parts of the organoid tissue when they penetrate it.
|
|
Now, Dr. Thomas Rauen's team, in collaboration with Dr. Peter Jones' team, has developed a novel microelectrode array system (Mesh-MEA) that not only provides optimal growth conditions for human brain organoids, but also enables non-invasive electrophysiological measurements throughout the growth period of the brain organoids (Biosensors and Bioelectronics, "A mesh microelectrode array for non-invasive electrophysiology within neural organoids").
|
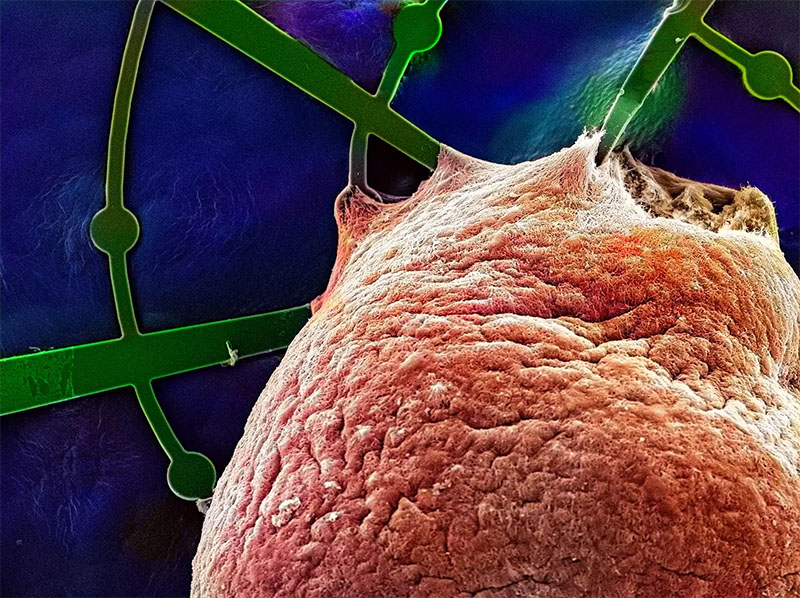 |
| A human brain organoid (colored red) grows on the hammock-like mesh structure of a Mesh-MEA (colored green). The scanning electron micrograph shows how the brain organoid has grown around the mesh filaments and microelectrodes (round structures in the green Mesh-MEA filaments). Most of the 61 microelectrodes are hidden in the bulk of the brain organoid. This human brain organoid grew on the Mesh-MEA for one year. (Image: Max Planck Institute for Molecular Biomedicine)
|
|
The scientists designed a kind of hammock for the brain organoids: "The hammock-like mesh structure provides 61 microelectrodes for electrophysiological measurements of neuronal network activity," explains Dr. Peter Jones.
|
|
The current study shows that brain organoids can not only be cultured on the newly developed Mesh MEA for up to one year but can also be continuously electrophysiologically analyzed during this period. "This is a great achievement because it allows us to study brain organoids for much longer than before. Normal human brain development takes a very long time, and neurodegenerative diseases also develop slowly," says Rauen.
|
|
The key to the current success is that the brain organoids enveloped the filaments and continued to grow on the spider web-like Mesh-MEA scaffold. Dr. Katherina Psathaki from CellNanOs at the University of Osnabrück was able to show this using an electron microscope. She analyzed brain organoids in their Mesh-MEA hammock one year after the start of cultivation. The images clearly confirm that the brain organoids develop in the suspended Mesh-MEA net structure. The microelectrodes are located in the center of the brain organoid tissue," adds Thomas Rauen.
|
|
The scientists observed spontaneous neuronal activity recorded by the microelectrodes in the brain organoids. "There was continuously recurring, synchronized neuronal activity throughout the recording phase, suggesting the formation of neuronal networks as seen in vivo," says Thomas Rauen.
|
|
Although brain organoids cannot represent all the functions of the human brain, Peter Jones and Thomas Rauen are convinced that the electrophysiological analysis of brain organoids using their newly developed Mesh-MEA system will open up the possibility of simulating specific functional aspects of human brain development and its diseases in the laboratory, which has not been possible until now.
|

