| Jun 23, 2023 |
Creating uniform DNA-encapsulating microgels that mimic a living cell
|
|
(Nanowerk News) The living cell harbors physiologically relevant components such as the genetic material (DNA) and proteins in a ‘self-organized’ setting. Understanding this process of self-assembly can reveal the underlying mechanism of self-organization of living matter. Water/oil (w/o) or water/water (w/w) droplets may be used as prototypes or “models” that mimic cells and can be used to study cellular self-assembly.
|
|
These models also have major implications in the field of biomedical research. Although cell mimetics can be generated using complicated and high-cost equipment, the associated methods are costly, tedious, and challenging.
|
|
Now, researchers from Japan have recently been able to develop a one-step method for producing uniform gelatin-based cell mimetics called “microgels.” The associated results were published in the journal Small ("Spontaneous Formation of Uniform Cell-Sized Microgels through Water/Water Phase Separation").
|
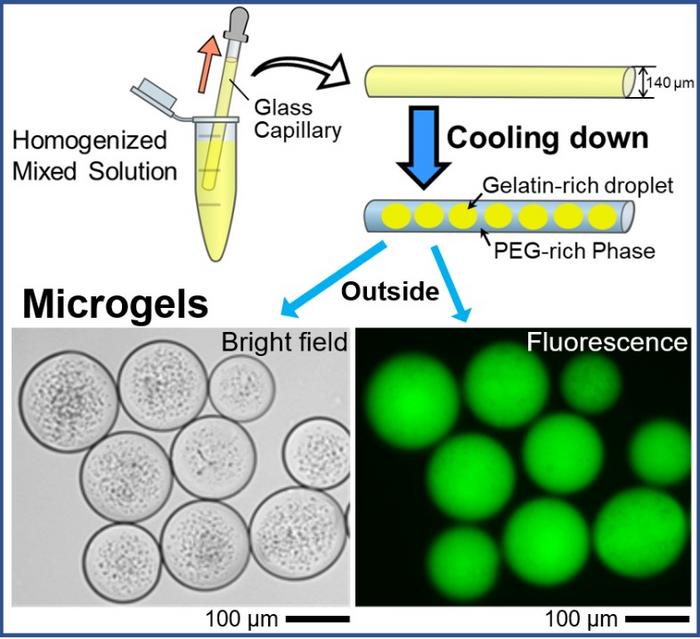 |
| By adapting aqueous polymer solution containing DNA, droplets entrapping DNA are generated in a self-organized manner through micro phase-separation, and these droplets are transformed into gel state by decreasing the temperature. The microgels are easily extruded into bulk water, maintaining their size. (Image: Akihisa Shioi, Doshisha University)
|
|
Explaining the motivation behind their study, Mayu Shono and Prof. Akihisa Shioi from Doshisha University, who led the study, remark, “Currently, our research focuses on understanding the self-organization of living matter. As an extension of our research activity, we have discovered an experimental procedure that may be quite useful for the generation of microgels.” The research team also comprised Gen Honda and Miho Yanagisawa of The University of Tokyo, and Kenichi Yoshikawa affiliated with Doshisha University and Kyoto University.
|
|
The mechanism of microgel formation is indeed interesting. The initial stage involves the generation of domain structures comprising of polyethylene glycol (PEG) and gelatin — two widely used synthetic crosslinkers. Decreasing the temperature to 24 °C favors the selective transition of the gelatin-rich domain into the gel phase. Under a defined set of experimental conditions, the PEG-rich phase migrates preferentially to the glass surface of the capillary tube owing to its higher affinity for glass and lower affinity for the gelatin-rich domains.
|
|
As a result, gelatin-rich droplets are engulfed by the PEG-rich phase. These findings were also validated in theoretical and numerical modelling studies using glass capillary experiments, which confirmed that the wettability of the inner surface of the glass capillary dominated w/w phase separation.
|
|
Moreover, up on the addition of DNA, the gelatin-rich droplets were able to spontaneously entrap DNA molecules owing to the phase separation of PEG and gelatin, giving rise to cell-mimicking microgels. The study also noted that the negatively charged DNA molecules incorporated in the droplets could stabilize them by preventing their fusion even above the sol/gel transition temperature.
|
|
The team also used a fluorescent dye to label and track the encapsulated DNA. Subsequent fluorescence microscopy experiments revealed the presence of round microgel structures harboring the glowing DNA molecules. According to the authors, the current approach is expected to confine, store, and transport huge DNA molecules within tiny cell-sized droplets!
|
|
Excited about the future scope of their research, PhD student Mayu Shono, the first author, says, “This novel method to form uniform cell-sized microgels may be applicable to other biopolymers. The uniform cell-sized and stable cell-like systems will also have key implications in the area of biological and life sciences.”
|
|
In summary, the study discusses a novel method for the preparation of gelatin-based cell mimetics, which can be tweaked to suit the desired purpose, depending on the area of application. “The method proposed in our study, which does not require special equipment, organic solvents, or surfactants, may be useful for producing microgels for food, medicines, cosmetics, and other materials,” Prof. Shioi concludes.
|

