| Oct 18, 2023 |
Unlocking the secrets of cell behaviour on soft substrates: A paradigm shift in mechanobiology
|
|
(Nanowerk News) A research group from the University of Turku and Turku Bioscience Centre together with Misvik Biology Ltd in Finland have developed a new method for studying how cancer cells function in softer and stiffer tissue environments. This insight challenges the existing paradigm, opening up new possibilities for research in cancer biology and tissue engineering.
|
Key Takeaways
|
|
The study disrupts long-held assumptions about cell growth, showing that cells can thrive in softer environments when given the right protein cues.
The study employs computational modeling and an array of growth conditions to analyze cell behavior in different stiffness levels with high resolution, overturning the notion that cells generally prefer stiffer surfaces for growth.
The research reveals that the right mix of proteins can facilitate cell growth on softer surfaces, paving the way for more accurate disease modeling and advancements in tissue engineering.
|
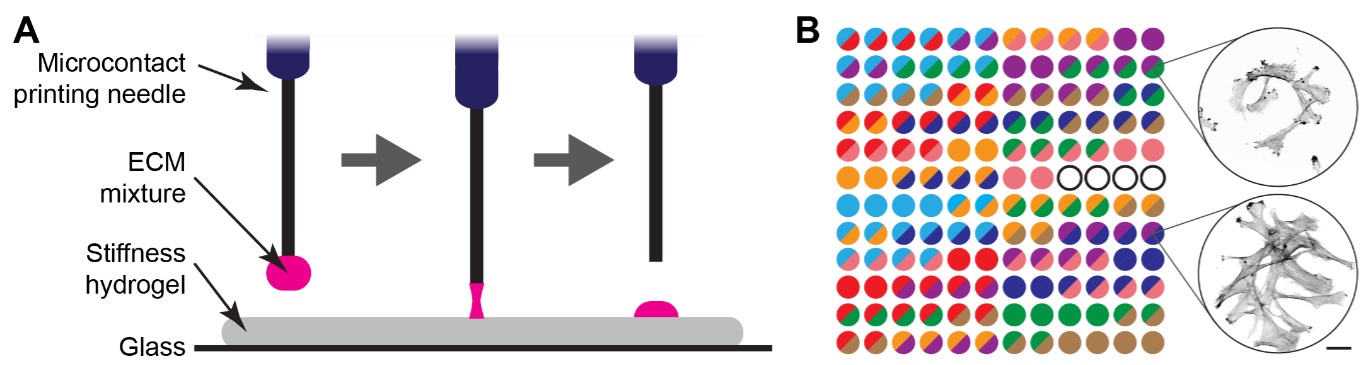 |
| Figure demonstrating the matrix spot array system. A: Schematic of the microcontact printing approach used to drop the protein mixtures on each spot. B: Each spot has a different protein mixture (scale bar: 50 µm). (Image: Turku Bioscience Centre, James Conway)
|
The Research
|
|
The findings have been published in PNAS ("Defined extracellular matrix compositions support stiffness-insensitive cell spreading and adhesion signaling").
|
|
A longstanding belief has been that cells outside the body prefer to spread and grow on stiffer surfaces. This is similar to when we walk on a concrete sidewalk (very stiff) and find it preferable to walking in mud (very soft). For this reason, cells, including stem cells, are continuously cultured on very stiff plastic or glass for research purposes.
|
|
This idea also resonates with cancer cells thriving within a hard lump they form in tissues. Usually, the stiffer the tumour, the poorer the patients’ prognosis. However, the stiffness of the tissues in our body (e.g., bone versus brain) is not the same. In fact, some cells like neurons and fat cells grow and function effectively in very soft surroundings.
|
|
The research group from the University of Turku and Turku Bioscience Centre collaborated with Misvik Biology Ltd, a biotechnology company based in Turku, Finland, to understand how cells function in softer environments and how these could be better modelled outside the human body. They used computational modelling and a large array of growth conditions to meticulously compare cell behaviour on soft and stiff surfaces at an unprecedented resolution.
|
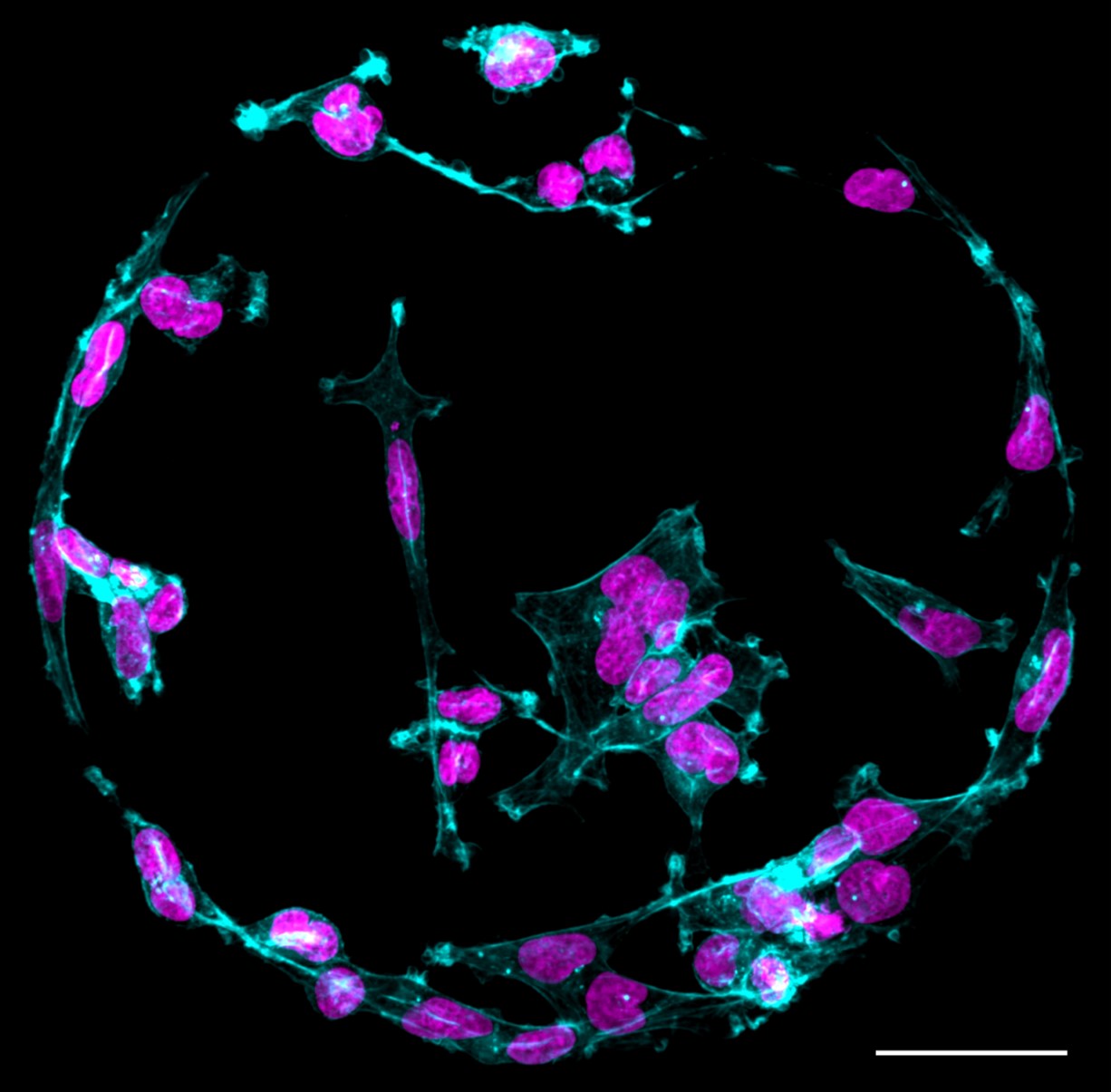 |
| Figure showing cancer cells on a printed protein spot. Osteosarcoma cells on protein spot. Scale bar: 50 µm. (Image: Turku Bioscience Centre, James Conway and Hellyeh Hamid)
|
New perspective on an old idea
|
|
Using an automated machine, the researchers microprinted different protein mixtures onto soft and stiff surfaces. The proteins chosen were those that typically surround cells in the body and give texture and information about the tissue environment.
|
|
“By using more diverse protein mixtures in cell culture, we begin to get closer to a more physiologically relevant setting outside the body with which we can more effectively model diseased and healthy states,” says Dr. James Conway from the InFLAMES research flagship, the lead researcher of the Turku Bioscience team.
|
|
“We had a specific system for microcontact printing of different protein mixtures onto culture plates and decided to see how we could expand the system. Once the researchers at Turku Bioscience had the idea, it was straightforward for us to then use the system on different stiffness hydrogels,” explains CEO Juha Rantala from Misvik, a key collaborator on the project.
|
|
By working together, the team demonstrated that the right combination of proteins can support cells on a soft surface, providing crucial survival cues that result in cell growth similar to that observed on a stiffer surface.
|
|
“These findings have unveiled a fundamental new perspective in mechanobiology. We have identified a mechanism through which we can explain how cells can effectively adhere, function, and signal on soft substrates. This insight challenges the existing paradigm, opening up new possibilities for research in cancer biology and tissue engineering,” notes Prof. Johanna Ivaska, the principal investigator of the project.
|


