| Dec 28, 2023 |
New plant-based scaffolds match animal alternatives for tissue engineering
|
|
(Nanowerk News) Tissue engineering seeks to repair damaged tissues by providing cellular scaffolds that support regeneration. Ideally, these scaffolds should be biocompatible, biodegradable materials that integrate with surrounding tissue. Currently, animal-derived proteins like gelatin serve as common scaffolding materials. However, recent research explores plant-based alternatives, which offer abundant availability, sustainability, and customizability.
|
|
So far, scaffolds built from animal collagen showed efficacy but faced sourcing limitations. Plant biopolymers now arise as candidate replacements but require compatibility assessments. A recent study in Advanced NanoBioMed Research ("Plant-Derived Zein as an Alternative to Animal-Derived Gelatin for Use as a Tissue Engineering Scaffold") evaluates corn zein protein versus standard gelatin for human stem cell scaffolds. Findings demonstrate zein’s stability, cell support capabilities, and in vivo compatibility - establishing viability as a gelatin substitute.
|
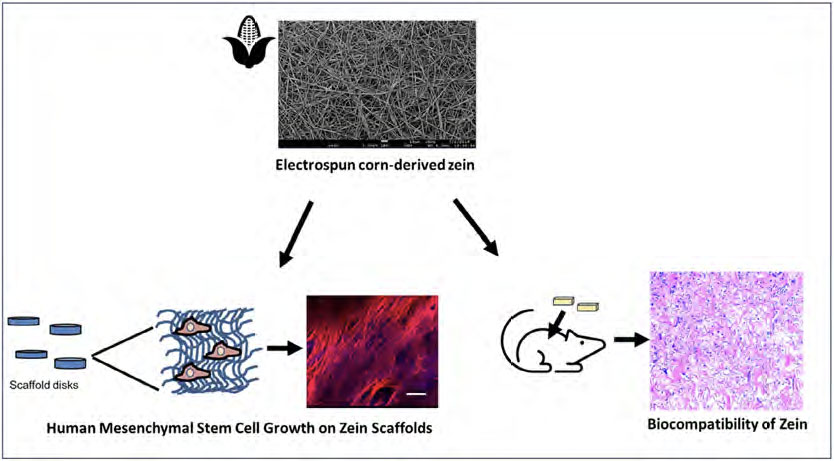 |
| Schematic illustration of the electrospun zein scaffold and its evaluation for use as a tissue engineering scaffold. Zein scaffolds supported human MSC growth, adhesion, and infiltration into the scaffold. Comparisons were performed with gelatin scaffolds. The zein scaffolds also supported the maintenance of MSC multipotency. The biocompatibility of the zein scaffolds with or without MSCs was also determined in a subcutaneous model. Findings demonstrate the potential of zein as a tissue engineering scaffold. (© Wiley-VCH Verlag)
|
Context Behind the Research
|
|
Tissue engineering emerged in the 1980s when cell biologists recognized that combining scaffold structures with living cells could repair damaged human tissues. Early research focused on bone and cartilage regeneration. The seminal concept involved seeding an implantable 3D structure with tissue-forming cells, then surgically introducing this construct to stimulate tissue regeneration in vivo.
|
|
Biocompatible and biodegradable scaffolds provide temporary stability and shape while transplanted or host cells deposit new extracellular matrix, gradually replacing the scaffold. Collagen proteins like gelatin performed well here due to native bioresorbability and innate cell adhesion molecules. However, researchers lacked fine control over animal-sourced scaffold degradation, hampering medical translations. Custom-engineered plant biopolymers now offer tailorable alternatives for tissue engineering scaffolds.
|
|
Zein protein from corn shows particular promise as its amino acid composition suits enzymatic biodegradation. Compared to gelatin, zein also confers superior scaffold stability as its hydrophobic residues resist hydration swelling - beneficial for maintaining structural integrity. Despite zein’s advantages, very few studies assessed feasibility as a lone scaffold component for tissue engineering. Addressing this gap, Limaye et al. fabricated zein-only fibrous scaffolds using an established electrospinning technique that creates mats mimicking native cellular matrix. Researchers then evaluated zein scaffold performance for human stem cell growth and tissue compatibility relative to standard gelatin controls.
|
Assessing Zein Scaffold Biostability
|
|
Initially assessing physical stability, tests showed zein scaffolds maintained structural integrity in fluid for over 3 weeks while swelling less than gelatin. Protein loss from scaffolds over this duration was comparable. These findings demonstrate zein electrospun into scaffolds provides stabilized structures for cell culture, overcoming previous instability limiting zein adoption.
|
Evaluating Stem Cell Interactions
|
|
Limaye et al next seeded scaffolds with human mesenchymal stem cells (MSCs) - multipotent progenitors that give rise to skeletal tissues. Over 2 weeks, microscopy revealed MSCs adhered, spread, and proliferated on zein fibers similarly to gelatin controls. Closer inspection found cells expressed key adhesion proteins like focal adhesion kinase and alpha-v-beta-3 integrin when attaching to either scaffold, enabling cell anchorage. Interestingly, cells migrated deeper into 3D zein scaffolds compared to gelatin, showing superior infiltration. This integration with surrounding structure benefits implant integration in intended tissue engineering applications.
|
Assessing In Vivo Response
|
|
The ultimate test for biomaterials involves surgical implantation to examine local tissue reactions. Here, researchers introduced stem cell-loaded zein scaffolds under the skin of mice, recovering the samples after 2 and 6 weeks. Similar to gelatin, histology revealed recruited cells populated scaffolds while signs of blood vessel in-growth supported integration. Scaffolds generally maintained fibrous morphology as cells deposited connective tissue matrix with no indications of immune rejection. Confirming zein biocompatibility and cell support potential in vivo, these results boost confidence for medical transition.
|
|
Importantly, upon extracting stem cells from zein scaffolds after 1 week then inducing lineage specification, cells retained multilineage differentiation capacity - demonstrating zein permits expanding stem cell populations while retaining progenitor potency required for subsequent tissue regeneration.
|
Realizing Plant-Based Tissue Scaffolds
|
|
Overall, this study uniquely positions zein as a custom-engineered, plant-sourced alternative to standard animal tissue scaffolds. Matching gelatin’s stem cell support while enabling stable electrospun structures, zein demonstrates biocompatibility required for regenerative medicine. These findings pave the way for further development, bringing society closer toward realizing abundant, sustainable scaffold materials for tissue engineering.
|

