| Apr 03, 2024 |
Must mRNA be cloaked in a lipid coat to serve as a vaccine?
|
|
(Nanowerk News) The Uchida Laboratory, led by Prof. Satoshi Uchida at the Department of Advanced Nanomedical Engineering, Medical Research Institute, Tokyo Medical and Dental University (TMDU), in collaboration with the Innovation Center of NanoMedicine (iCONM), Tokyo Metropolitan Institute of Medical Science, Kyorin University, and NANO MRNA Co., Ltd., has demonstrated that intradermal administration of naked mRNA, without the protection of nanoparticles, induces robust vaccination against SARS-CoV-2, the virus responsible for COVID-19, in both mice and primates.
|
|
This groundbreaking study is the first to report a successful naked mRNA vaccine that prevents SARS-CoV-2 infection without the use of lipid nanoparticles (LNPs), which are known to cause systemic adverse events.
|
|
The vaccine is currently under development for clinical trials, and the detailed research results will are published in the international medical journal Molecular Therapy ("Carrier-free mRNA vaccine induces robust immunity against SARS CoV-2 in mice and non-human primates without systemic reactogenicity").
|
 |
| Schematic diagram illustrating jet injection of a "Naked mRNA" vaccine. (Image: Satoshi Uchida)
|
|
During the COVID-19 pandemic, mRNA vaccines have proven to be highly effective, with billions of doses administered worldwide. However, their rapid development has led to challenges, particularly concerning relatively strong adverse reactions, including severe ones. While these adverse reactions may be considered acceptable for a limited number of doses during a pandemic, a safer platform that allows multiple doses over a lifetime is desirable for ongoing COVID-19 boosters and the extension of mRNA vaccine applications to other infectious diseases.
|
|
The adverse reactions associated with current mRNA vaccines are primarily attributed to the LNPs that carry the mRNA. LNPs possess immunostimulatory properties and can spill out of the injection site, leading to systemic inflammatory responses. Nonetheless, LNPs play crucial roles in vaccine efficacy, such as preventing mRNA degradation, efficiently delivering mRNA into cells, migrating to lymph nodes to deliver mRNA into immune cells, and stimulating the immune system through immunostimulatory lipids. The present study aims to obtain these functions without relying on LNPs.
|
|
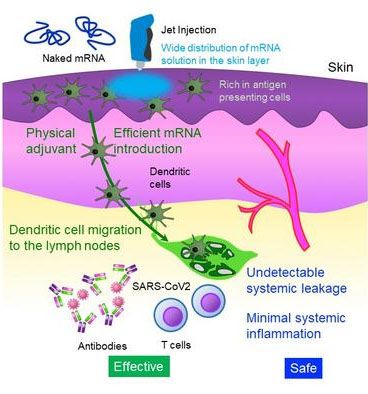
This study provides a straightforward and safe design, utilizing the administration of "naked mRNA." To target immune cells more effectively, the researchers focused on the skin tissue, which is more abundant in immune cells compared to muscle tissue, the current administration site of mRNA vaccines.
|
|
Additionally, the research team used a "Jet Injector" that facilitates mRNA delivery to skin cells by utilizing the physical stress induced by jet flow. In a reporter study, the "Jet Injector" improved mRNA delivery efficiency by more than 100-fold compared to conventional needle and syringe injection, and the mRNA remained at the injection site without detectable systemic leakage. In contrast, mRNA-loaded LNPs migrated to the liver, spleen, and other systemic organs after intradermal administration, provoking inflammation in those areas. Furthermore, inflammation at the injection site was minimal with the "naked mRNA" method, whereas "mRNA cloaked in a lipid coat" induced infiltration of inflammatory cells and tissue necrosis.
|
 |
| Jet Injector used for the injection of "Naked mRNA". (Image: Satoshi Uchida)
|
|
The research team first demonstrated the vaccination ability of "naked mRNA" using a model antigen. The jet injector drastically improved the efficacy of antibody production to a level comparable to that of "mRNA cloaked in a lipid coat" at the maximum tolerable doses. These antibodies combat viruses in the body, preventing infection, but they cannot remove infected cells. On the other hand, cellular immunity removes such diseased cells, playing a critical role in preventing severe diseases.
|
|
Intriguingly, the "naked mRNA vaccine" effectively increases the number of immunocytes, such as CD4-positive T cells and CD8-positive T cells. The research team then conducted virus challenge experiments after the "naked mRNA" vaccination targeting the spike protein of the SARS-CoV-2 virus. The vaccination significantly lowered the amount of virus in the lungs and alleviated lung inflammation compared to an unvaccinated control. This vaccine provided cynomolgus monkeys with vaccine efficacy comparable to that of mice without significant adverse reactions.
|
|
The study also includes mechanistic analyses. The "naked mRNA vaccine" stayed at the injection site and did not migrate to the lymph nodes. Instead, antigen-presenting cells that took up mRNA at the injection site migrated to the lymph nodes, contributing to the vaccination efficacy. The vaccine induced the maturation of the lymph node near the injection site. The "Jet Injector" caused transient inflammation localized to the injection site, recruiting lymphocytes, which may function as a "Physical Adjuvant" to enhance vaccination efficacy. These local inflammatory reactions disappeared within a few days.
|
|
In conclusion, the "naked mRNA vaccine" reduces systemic adverse reactions, an issue with "mRNA cloaked in a lipid coat," and induces immunity sufficient for the protection from infectious diseases. This world-leading achievement in preventing infectious diseases with mRNA alone may lead to a platform that allows repeated dosing with minor adverse reactions. Further studies are being conducted, with the aim of a clinical trial planned for 2026.
|


