| Dec 01, 2016 |
New process produces hydrogen at much lower temperature
|
|
(Nanowerk News) Waseda University researchers have developed a new method for producing hydrogen, which is fast, irreversible, and takes place at much lower temperature using less energy (Scientific Reports, "Surface Protonics Promotes Catalysis"). This innovation is expected to contribute to the spread of fuel cell systems for automobiles and homes.
|
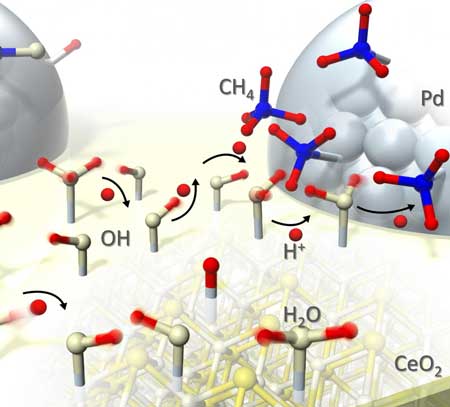 |
| This is an illustration of proton hopping during catalytic reaction. (Image: Waseda University)
|
|
Hydrogen has normally been extracted from methane and steam using a nickel catalyst at temperatures of over 700°C. However, the high temperature creates major challenges for widespread use.
|
|
The group led by Professor Yasushi Sekine, Waseda University Faculty of Science and Engineering, developed a method which allows hydrogen extraction at temperatures as low as 150~200°C. This shift greatly reduces energy input needed to produce hydrogen fuel, extends catalyst life, reduces the cost of construction materials, and reduces complexity of heat-management (cooling) systems.
|
|
Although the research group had already seen that a fast reaction would be possible even in the range of 150~200°C by applying a weak electric field (surface protonics), the mechanism had not been fully understood.
|
|
In this research, the group is the first to explain the mechanism by observing the catalyst during reaction. Protons move quickly through water adsorbed on the catalyst's surface, and protons' surface "hopping" allows reaction to proceed at low temperatures. Furthermore, the collision of the protons and the adsorbates prevents reversal of the reaction.
|
|
As momentum grows for the commercialization of hydrogen, this research is not only applicable to hydrogen production, but also to many consumer products since the same mechanism makes it possible to lower the temperature for various reactions involving hydrogen or water. The process is already being applied to research for improving energy efficiency in automobiles by creating reactions between exhaust gases and fuel at low temperature.
|

