| Nov 04, 2019 |
Post-lithium battery technology
|
|
(Nanowerk News) Next-generation batteries will probably see the replacement of lithium ions by more abundant and environmentally benign alkali metal or multivalent ions. A major challenge, however, is the development of stable electrodes that combine high energy densities with fast charge and discharge rates.
|
|
In the journal Angewandte Chemie ("A Pyrazine-Based Polymer for Fast-Charge Batteries"), US and Chinese scientists report a high-performance cathode made of an organic polymer to be used in low-cost, environmentally benign, and durable sodium-ion batteries.
|
 |
| A Pyrazine-Based Polymer for Fast-Charge Batteries. (© Wiley)
|
|
Lithium-ion batteries are the state-of-the-art technology for portable devices, energy storage systems, and electric vehicles, the development of which has been awarded with this year’s Nobel prize. Nevertheless, next-generation batteries are expected to provide higher energy densities, better capacities, and the usage of cheaper, safer, and more environmentally benign materials.
|
|
New battery types that are most explored employ essentially the same rocking-chair charging–discharging technology as the lithium battery, but the lithium ion is substituted with cheap metal ions such as sodium, magnesium, and aluminum ions. Unfortunately, this substitution brings along major adjustments to the electrode materials.
|
|
Organic compounds are favorable as electrode materials because, for one, they do not contain harmful and expensive heavy metals, and they can be adapted to different purposes. Their disadvantage is that they dissolve in liquid electrolytes, which makes electrodes inherently unstable.
|
|
Chunsheng Wang and his team from the University of Maryland, USA, and an international team of scientists have introduced an organic polymer as a high-capacity, fast-charging, and insoluble material for battery cathodes. For the sodium ion, the polymer outperformed current polymeric and inorganic cathodes in capacity delivery and retention, and for multivalent magnesium and aluminum ions, the data did not lag far behind, according to the study.
|
|
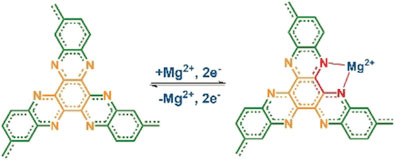
As a suitable cathodic material, the scientists identified the organic compound hexaazatrinaphthalene (HATN), which has already been tested in lithium batteries and supercapacitors, where it functions as a high-energy-density cathode that rapidly intercalates lithium ions.
|
|
However, like most organic materials, HATN dissolved in the electrolyte and made the cathode unstable during cycling. The trick was now to stabilize the material’s structure by introducing linkages between the individual molecules, the scientists explained. They obtained an organic polymer called polymeric HATN, or PHATN, which offered fast reaction kinetics and high capacities for sodium, aluminum, and magnesium ions.
|
|
After assembling the battery, the scientists tested the PHATN cathode using a high-concentrated electrolyte. They found excellent electrochemical performances for the non-lithium ions. The sodium battery could be operated at high voltages up to 3.5 volts and maintained a capacity of more than 100 milliampere hours per gram even after 50 000 cycles, and the corresponding magnesium and aluminum batteries were close behind these competitive values, reported the authors.
|
|
The researchers envision these polymeric pyrazine-based cathodes (pyrazine is the organic substance upon which HATN is based; it is an aromatic benzol-like, nitrogen-rich organic substance with a fruity flavor) to be employed in environmentally benign, high-energy-density, fast and ultrastable next generation rechargeable batteries.
|

