| Aug 03, 2023 |
New photocatalytic system converts carbon dioxide to valuable fuel more efficiently than natural photosynthesis
|
|
(Nanowerk News) A joint research team from City University of Hong Kong (CityU) and collaborators recently developed a stable artificial photocatalytic system that is more efficient than natural photosynthesis. The new system mimics a natural chloroplast to convert carbon dioxide in water into methane, a valuable fuel, very efficiently using light. This is a promising discovery, which could contribute to the goal of carbon neutrality.
|
|
Photosynthesis is the process by which chloroplasts in plants and some organisms use sunlight, water and carbon dioxide to create food or energy. In past decades, many scientists have tried to develop artificial photosynthesis processes to turn carbon dioxide into carbon-neutral fuel.
|
|
“However, it is difficult to convert carbon dioxide in water because many photosensitizers or catalysts degrade in water,” explained Professor Ye Ruquan, Associate Professor in the Department of Chemistry at CityU, one of the leaders of the joint study (Nature Catalysis, "Artificial spherical chromatophore nanomicelles for selective CO2 reduction in water"). “Although artificial photocatalytic cycles have been shown to operate with higher intrinsic efficiency, the low selectivity and stability in water for carbon dioxide reduction have hampered their practical applications.”
|
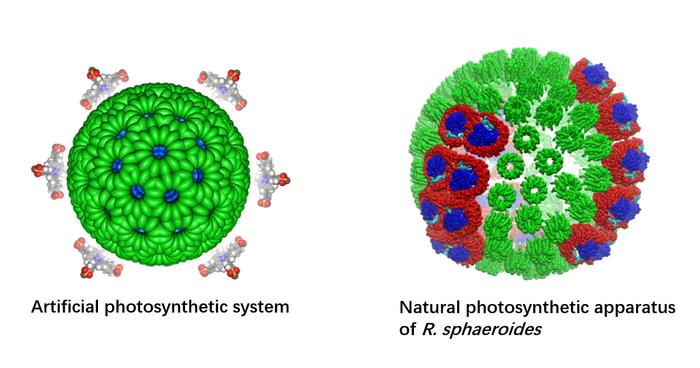 |
| (Image: (left) Professor Ye Ruquan’s research group / City University of Hong Kong and (right) Biophysical Journal, 99:67-75, 2010)
|
|
In the latest study, the joint-research team from CityU, The University of Hong Kong (HKU), Jiangsu University and the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences overcame these difficulties by using a supramolecular assembly approach to create an artificial photosynthetic system. It mimics the structure of a purple bacteria’s light-harvesting chromatophores (i.e. cells that contain pigment), which are very efficient at transferring energy from the sun.
|
|
The core of the new artificial photosynthetic system is a highly stable artificial nanomicelle – a kind of polymer that can self-assemble in water, with both a water-loving (hydrophilic) and a water-fearing (hydrophobic) end. The nanomicelle’s hydrophilic head functions as a photosensitizer to absorb sunlight, and its hydrophobic tail acts as an inducer for self-assembly. When it is placed in water, the nanomicelles self-assemble due to intermolecular hydrogen bonding between the water molecules and the tails. Adding a cobalt catalyst results in photocatalytic hydrogen production and carbon dioxide reduction, resulting in the production of hydrogen and methane.
|
|
Using advanced imaging techniques and ultrafast spectroscopy, the team unveiled the atomic features of the innovative photosensitizer. They discovered that the special structure of the nanomicelle’s hydrophilic head, along with the hydrogen bonding between water molecules and the nanomicelle’s tail, make it a stable, water-compatible artificial photosensitizer, solving the conventional instability and water-incompatibility problem of artificial photosynthesis. The electrostatic interaction between the photosensitizer and the cobalt catalyst, and the strong light-harvesting antenna effect of the nanomicelle improved the photocatalytic process.
|
|
In the experiment, the team found that the methane production rate was more than 13,000 µmol h−1 g−1, with a quantum yield of 5.6% over 24 hours. It also achieved a highly efficient solar-to-fuel efficiency rate of 15%, surpassing natural photosynthesis.
|
|
Most importantly, the new artificial photocatalytic system is economically viable and sustainable, as it doesn’t rely on expensive precious metals. “The hierarchical self-assembly of the system offers a promising bottom-up strategy to create a precisely controlled, high-performance artificial photocatalytic system based on cheap, Earth-abundant elements, like zinc and cobalt porphyrin complexes,” said Professor Ye.
|
|
Professor Ye said he believes the latest discovery will benefit and inspire the rational design of future photocatalytic systems for carbon dioxide conversion and reduction using solar energy, contributing to the goal of carbon neutrality.
|

